Gamma-hydroxybutyrate (GHB) is a naturally occurring neurochemical and a powerful central nervous system depressant. In medicine, its prescription form—oxybate—is used under strict supervision to treat narcolepsy with cataplexy, excessive daytime sleepiness, and idiopathic hypersomnia. Unlike many sleep-related products marketed as supplements, GHB is not an over-the-counter aid; it is a controlled prescription therapy with significant safety requirements and a well-documented potential for harm when misused. When used appropriately, oxybate can consolidate nighttime sleep, deepen slow-wave sleep, and reduce cataplexy and daytime sleepiness. However, dosing is precise, monitoring is essential, and interactions—especially with alcohol and other sedatives—can be life-threatening. This guide explains what GHB is and is not, how the medical products work, where the clinical evidence is strongest, practical considerations for supervised use, and the side effects and risks you should understand before any discussion with your clinician.
Key Insights
- Strongest evidence: prescription oxybate improves cataplexy and daytime sleepiness in narcolepsy and benefits idiopathic hypersomnia symptoms.
- Never combine with alcohol, benzodiazepines, opioids, or other sedatives; respiratory depression and death can occur.
- Typical medical dosing: immediate-release 4.5–9 g per night in two doses; extended-release 6–9 g once nightly—prescription-only and clinician-supervised.
- Avoid outside medical care; also avoid in pregnancy, untreated sleep-disordered breathing, and active substance use disorders without specialist oversight.
Table of Contents
- What is GHB, really?
- Does it work? What the medical evidence shows
- How doctors prescribe and monitor oxybate
- How much GHB per day? (medical dosing only)
- Side effects, interactions, risks, and precautions
- GHB versus alternatives for narcolepsy and hypersomnia
What is GHB, really?
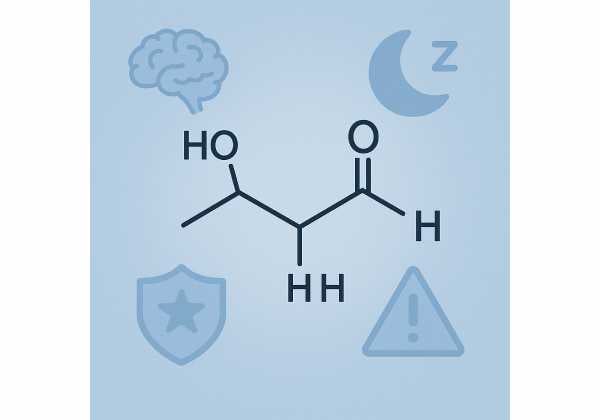
Gamma-hydroxybutyrate (GHB) is both an endogenous molecule found in the brain and a synthetic drug with potent sedative effects. In medical practice, you will encounter it as oxybate—an oral solution of the sodium salt (sodium oxybate) or mixed salts (lower-sodium oxybate). These prescription products are indicated for narcolepsy (to reduce cataplexy and excessive daytime sleepiness) and, more recently, for idiopathic hypersomnia (IH). A once-nightly extended-release sodium oxybate is also available for narcolepsy.
Mechanistically, oxybate enhances slow-wave sleep and restructures nocturnal sleep architecture. It acts primarily as a GABAB receptor agonist at therapeutic concentrations and also interacts with high-affinity GHB receptors. Clinically, this translates to more consolidated nighttime sleep, fewer cataplexy attacks, and reduced daytime sleepiness in appropriately selected patients.
A few critical distinctions:
- Not a supplement. GHB is a controlled medication. Products sold online or in gyms as “G,” “liquid ecstasy,” or “natural” sleep aids are dangerous and illegal in many jurisdictions.
- Different formulations, different logistics.
- Immediate-release (IR) oxybate is taken twice nightly (one dose at bedtime, a second 2.5–4 hours later).
- Extended-release (ER) oxybate is taken once nightly.
- Lower-sodium oxybate provides the same active oxybate with substantially reduced sodium content per nightly dose, which may matter for people managing cardiovascular or renal risk.
- Why it helps narcolepsy and IH: By deepening slow-wave sleep and stabilizing nighttime rest, oxybate can lessen daytime symptom burden and cataplexy frequency in narcolepsy, and reduce sleep inertia and excessive sleepiness in IH.
Because GHB has a narrow safety margin, all use should occur within a clinician-directed treatment plan with careful education, monitoring, and avoidance of contraindicated substances. The remainder of this guide focuses on supervised, medical use—not recreational or unsupervised use.
Does it work? What the medical evidence shows
For narcolepsy, multiple randomized and controlled studies—spanning immediate-release and extended-release oxybate—show consistent improvement in excessive daytime sleepiness, cataplexy frequency, and patient-reported outcomes. A widely cited clinical practice guideline from the American Academy of Sleep Medicine (AASM) recommends sodium oxybate for narcolepsy in adults and supports its use in pediatric narcolepsy under appropriate conditions. These recommendations are grounded in several trials demonstrating meaningful reductions in weekly cataplexy episodes and improved wakefulness measures.
In idiopathic hypersomnia (IH), a phase 3 randomized, placebo-controlled, double-blind withdrawal study of lower-sodium oxybate demonstrated clinically meaningful improvements in Epworth Sleepiness Scale scores and other symptom measures during the double-blind period following individualized open-label titration. Participants randomized to continue oxybate maintained benefits, while those switched to placebo worsened, indicating the treatment effect was not simply regression to the mean. Safety signals were consistent with the established profile (e.g., nausea, dizziness, headache), without new unexpected concerns in the controlled setting.
For extended-release once-nightly sodium oxybate in narcolepsy, the pivotal REST-ON trial reported significant improvements across co-primary endpoints (maintenance of wakefulness, clinician global impression, and cataplexy frequency) compared with placebo, using once-nightly doses titrated across 6 g, 7.5 g, and 9 g at bedtime. Subsequent analyses have reinforced efficacy across narcolepsy type 1 and type 2 and described improvements in disrupted nighttime sleep, an often overlooked driver of daytime impairment.
Beyond outcomes, pharmacology research clarifies why oxybate works in these conditions: it increases slow-wave sleep, reduces sleep fragmentation, and modulates neurotransmission through GABAergic pathways. The half-life is short (about 30–60 minutes), which explains the traditional two-dose nightly approach for immediate-release products and the rationale for extended-release technology to maintain therapeutic levels overnight.
What the data do not show: oxybate is not a stimulant and does not cure narcolepsy or IH. Benefits depend on adherence, correct timing, and co-management (e.g., behavioral sleep strategies, safe schedules). Also, oxybate’s benefits for conditions outside approved indications (e.g., general insomnia, performance enhancement) are not supported and its risk profile makes such use inappropriate.
Taken together, the evidence base supports oxybate as a core therapy for narcolepsy (particularly with cataplexy) and a validated option for IH—when prescribed, counseled, and monitored carefully.
How doctors prescribe and monitor oxybate
Eligibility and evaluation. Clinicians confirm the diagnosis (e.g., polysomnography and multiple sleep latency testing for narcolepsy; vetted criteria for IH) and screen for factors that raise risk: sleep-disordered breathing, pulmonary disease, hepatic impairment, psychiatric comorbidity, substance use disorders, and potential drug–drug interactions. A careful medication and alcohol history is mandatory.
Choosing a formulation.
- Immediate-release (two nightly doses). Suitable for many patients; requires waking to take a second dose.
- Extended-release (once nightly). Offers a single bedtime dose, eliminating nocturnal awakening to re-dose.
- Lower-sodium oxybate. Reduces nightly sodium load compared with traditional sodium oxybate—relevant for patients managing blood pressure, heart failure risk, or renal concerns.
Starting, titrating, and scheduling (under prescription only).
- Immediate-release commonly starts at 4.5 g per night split into 2.25 g at bedtime and 2.25 g 2.5–4 hours later, then titrates by up to 1.5 g per night per week to a 6–9 g per night target range based on response and tolerability.
- Extended-release uses once-nightly bedtime dosing, generally titrated across 6 g → 7.5 g → 9 g in clinical trials.
- The bedtime dose should be taken while in bed, and patients typically fall asleep within minutes. If the second immediate-release dose is missed, it is not taken later that night.
Education that patients receive.
- No alcohol, no sedatives, no opioids, no benzodiazepines. Combining oxybate with other depressants can cause respiratory depression, loss of consciousness, and death.
- Nighttime safety. Take doses in bed, plan for uninterrupted sleep, and allow adequate time (e.g., at least 6 hours) before engaging in activities requiring alertness the next morning (such as driving).
- Storage and handling. Keep only in original, child-resistant containers; measure doses precisely; never share or stockpile.
- Follow-up. Regular visits assess cataplexy frequency, sleepiness scores, adverse effects, and any hints of misuse or diversion.
Special scenarios.
- Sleep-disordered breathing. Oxybate may affect respiratory drive; patients with obstructive sleep apnea typically require treatment (e.g., PAP therapy) and careful monitoring.
- Hepatic impairment. Lower starting doses are generally used, with cautious titration.
- Pediatrics. Select pediatric patients with narcolepsy may be candidates under specialist care; child-specific weight-based regimens and close monitoring apply.
- Pregnancy and lactation. Human data are limited; decisions require individualized risk–benefit assessment and often favor alternatives.
When to pause or stop. Worsening depression, suicidality, new or severe parasomnias, troublesome nausea or dizziness despite adjustment, persistent sleep-disordered breathing concerns, or any suggestion of misuse generally prompt reevaluation and, if needed, discontinuation with clinician oversight.
How much GHB per day? (medical dosing only)
Important: The following reflects prescription-only, clinician-directed dosing for approved oxybate products. Do not attempt to self-dose or use any non-medical GHB; the risk of overdose and death is high.
Immediate-release (two nightly doses). Many adults start at 4.5 g per night divided into two equal doses (2.25 g at bedtime and 2.25 g 2.5–4 hours later). Clinicians typically increase by up to 1.5 g per night each week (for example, adding 0.75 g to each dose) to the 6–9 g per night range, guided by cataplexy frequency, daytime sleepiness, tolerability, and comorbidities. Doses above 9 g per night are generally not used. Some patients do better with unequal split doses (e.g., a slightly larger first dose).
Extended-release (once nightly). Clinical trials in narcolepsy have used 6 g → 7.5 g → 9 g once nightly, titrated based on response and side effects. The once-nightly schedule removes the need to wake for a second dose and may reduce sleep disruption.
Idiopathic hypersomnia (IH). For lower-sodium oxybate in IH, dosing is individualized across similar total nightly amounts, with once-nightly, twice-nightly, or (less often) thrice-nightly regimens used during optimization in studies. A clinician decides the pattern based on symptom profile (e.g., sleep inertia) and tolerability.
With food? For immediate-release products, the first dose is taken at least 2 hours after eating to reduce variability in absorption. The second dose is taken on schedule, not with food. Extended-release is taken at bedtime; follow prescriber instructions.
Dose adjustments and special populations.
- Hepatic impairment: Lower starting dose and cautious titration.
- Pediatrics (narcolepsy): Weight-based dosing under pediatric sleep specialist care.
- Older adults: Start low, go slow; monitor for falls, confusion, or respiratory issues.
Never do the following:
- Do not double a dose to “catch up.”
- Do not take a second immediate-release dose if you slept through the planned time; skip it and resume the next night.
- Do not combine with alcohol, benzodiazepines, opioids, barbiturates, sedating antihistamines, or other sleep medications.
The guiding principle with oxybate is precision: exact measuring, exact timing, and regular follow-up. Any dose changes should be clinician-directed after reviewing benefits, side effects, and life circumstances (e.g., shift work, travel).
Side effects, interactions, risks, and precautions
Common side effects (often dose-related or improving with time and titration): nausea, vomiting, dizziness, headache, enuresis, anxiety, and tremor. Some patients report increased sweating, parasomnias, or confusional arousals (e.g., sleepwalking). Clinicians often mitigate these by slowing titration, adjusting split-dose ratios, or changing formulations.
Serious risks and boxed warnings (medical products).
- Respiratory depression and obtundation can occur even at prescribed doses. Risk rises sharply when combined with alcohol or other CNS depressants (benzodiazepines, opioids, barbiturates, sedatives, or certain muscle relaxants).
- Abuse, misuse, and diversion. Oxybate contains the same active oxybate ion as GHB. A careful assessment for substance use disorders, ongoing monitoring, and secure storage are essential.
- Sleep-disordered breathing. Oxybate can worsen central apneas and oxygen desaturation in susceptible individuals. Treatment of obstructive sleep apnea and close follow-up are crucial.
- Neuropsychiatric reactions. Depression, suicidality, agitation, confusion, and hallucinations can occur. Any new or worsening psychiatric symptom warrants prompt medical review.
- Rare but important: seizures, allergic reactions, and significant parasomnias.
Withdrawal and dependence. Chronic high-dose or unsupervised GHB use (outside medical care) can produce severe withdrawal characterized by anxiety, tremor, insomnia, autonomic instability, and delirium that may require inpatient management. This is another reason unsupervised or illicit use is dangerous and must be avoided.
Absolute do-not-mix list.
- Alcohol (beer, wine, spirits)
- Benzodiazepines (e.g., diazepam, clonazepam)
- Opioids (e.g., oxycodone, hydromorphone, methadone)
- Barbiturates
- Sedating antidepressants/antipsychotics (risk-based clinician judgment applies)
- Other sleep medicines or sedating antihistamines
Who should not use or needs specialist clearance first:
- Pregnancy or breastfeeding (insufficient data; weigh risks carefully)
- Untreated sleep apnea or significant hypoventilation
- Severe hepatic impairment (requires dose modification and specialist oversight)
- Active substance use disorder or high diversion risk
- Unreliable medication adherence (e.g., inability to safely measure, store, or schedule doses)
Emergency guidance. If someone on oxybate becomes unrouseable, has slowed or stopped breathing, or vomits while unconscious, call emergency services immediately. Place the person in the recovery position if safe to do so and do not give additional substances.
Finally, remember that medical oxybate is only appropriate within a structured treatment plan. Any non-prescribed, recreational, or opportunistic use is dangerous and, in many places, illegal.
GHB versus alternatives for narcolepsy and hypersomnia
Choosing therapy for narcolepsy or IH is rarely a one-drug decision. The goal is a tailored regimen that addresses nighttime sleep, daytime wakefulness, and cataplexy (when present), balanced against risks, preferences, and lifestyle.
Where oxybate fits.
- In narcolepsy, clinical guidelines recommend sodium oxybate as a mainstay for cataplexy and daytime sleepiness, often alongside wake-promoting agents. Patients with pronounced disrupted nighttime sleep or frequent cataplexy may see the most benefit.
- In idiopathic hypersomnia, lower-sodium oxybate is an evidence-based option that can improve sleep inertia and daytime symptoms after individualized titration.
How it compares to other options:
- Modafinil/armodafinil. First-line wake-promoting agents for many patients; improve daytime alertness but do not treat cataplexy. Typically well-tolerated; watch for headaches, anxiety, and rare rash.
- Solriamfetol. Dual DAT/NET reuptake inhibitor that increases wakefulness; neutral for cataplexy; blood pressure and heart-rate monitoring are standard.
- Pitolisant. Histamine H3 antagonist/inverse agonist that improves wakefulness and can reduce cataplexy. Non-sedating; watch for insomnia and QT considerations.
- Traditional stimulants (methylphenidate, amphetamines). Effective for wakefulness; potential for tolerance, appetite suppression, cardiovascular effects.
- Behavioral strategies. Scheduled naps (narcolepsy), consistent sleep–wake times, treatment of sleep apnea, careful caffeine timing, and light/activity planning meaningfully augment pharmacologic therapy.
Pros of oxybate. Addresses multiple symptom domains (nighttime consolidation, cataplexy, daytime sleepiness) and can reduce the need for multiple medications. Once-nightly ER may improve convenience. Lower-sodium options can be advantageous for cardiometabolic risk.
Cons and cautions. Narrow therapeutic margin, serious interaction risks, night-time dosing logistics (for immediate-release), and potential misuse/diversion. Some patients experience limiting nausea or dizziness despite gradual titration.
Practical selection logic (with your clinician):
- Primary goals include cataplexy control or severe disrupted nighttime sleep → consider oxybate early.
- Primary goal is daytime alertness without cataplexy → consider modafinil/armodafinil, solriamfetol, or pitolisant, with oxybate as an add-on if sleep consolidation is needed.
- Complex comorbidities (e.g., cardiovascular disease, respiratory compromise, significant psychiatric history) → weigh lower-sodium formulations, alternatives, and non-pharmacologic strategies carefully.
The best outcomes come from integrated care: a clear diagnosis, realistic goal-setting, the right medication(s) at the right dose, and consistent follow-up to fine-tune treatment while safeguarding safety.
References
- Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline (2021) (Guideline)
- Safety and efficacy of lower-sodium oxybate in adults with idiopathic hypersomnia: a phase 3, placebo-controlled, double-blind, randomised withdrawal study (2022) (RCT)
- Once-nightly sodium oxybate (FT218) demonstrated improvement of symptoms in a phase 3 randomized clinical trial in patients with narcolepsy (2022) (RCT)
- γ-Hydroxybutyric Acid: Pharmacokinetics, Pharmacodynamics, and Toxicology (2021) (Systematic Review/Narrative Review)
- Xyrem (sodium oxybate) Information (2017)
Medical Disclaimer
This article provides general information and is not a substitute for medical advice. GHB/oxybate products are prescription-only therapies with significant risks and strict safety requirements. Do not start, stop, or change any medication based on this content. Decisions about diagnosis, eligibility, dosing, and monitoring must be made with a qualified clinician who knows your medical history. If you or someone else has taken GHB outside medical supervision or is experiencing symptoms of overdose (trouble breathing, unresponsiveness), call emergency services immediately.
If this guide was useful, please consider sharing it on Facebook, X (formerly Twitter), or any platform you prefer, and follow us for future evidence-based articles. Your support helps us continue creating careful, trustworthy health content.