In the ever-evolving landscape of ocular therapies, Verkazia stands out as a groundbreaking treatment designed to address severe inflammation with modern cyclosporine emulsion. With innovative formulation and delivery methods, Verkazia offers renewed hope to those who have long struggled with the discomfort and visual impairment associated with vernal keratoconjunctivitis. This modern cyclosporine emulsion is formulated to provide targeted anti-inflammatory effects, aiming to restore ocular surface balance and improve overall eye health. Its unique composition not only optimizes the absorption of cyclosporine into the ocular tissues but also minimizes common side effects that have historically limited the use of traditional formulations.
Imagine a therapy that combines advanced drug delivery technology with potent immunomodulatory effects to provide relief from the relentless irritation and inflammation that can affect your daily life. Verkazia is that therapy. By harnessing the power of cyclosporine in an emulsion form, Verkazia addresses the inflammatory cascade at its root, calming the overactive immune responses that characterize severe ocular surface inflammation. The formulation has been meticulously engineered to ensure that the active ingredient reaches its target tissues effectively, thereby reducing the frequency of flare-ups and the overall burden of treatment.
What makes Verkazia particularly compelling is its dual focus on efficacy and patient comfort. While traditional treatments for vernal keratoconjunctivitis may require frequent dosing and can lead to discomfort upon application, Verkazia is designed with patient convenience in mind. Its modern emulsion technology enhances the bioavailability of cyclosporine, meaning that patients may experience a more rapid onset of relief without the irritation often seen with older formulations. This is especially beneficial for individuals who need a long-term solution to manage their symptoms while maintaining an active lifestyle.
The innovation behind Verkazia is rooted in years of research and clinical refinement. By optimizing the formulation of cyclosporine into a stable, effective emulsion, researchers have been able to overcome many of the challenges that previously hindered the therapeutic use of cyclosporine in ocular treatments. The emulsion is designed to provide a sustained release of the active compound, ensuring that therapeutic levels are maintained over an extended period. This not only reduces the need for frequent applications but also minimizes the fluctuations in drug concentration that can lead to inconsistent therapeutic outcomes.
Moreover, Verkazia’s formulation is supported by a robust framework of scientific evidence and clinical experience. Early adopters of this therapy have reported significant improvements in ocular comfort, reduced redness, and an overall decrease in the severity of inflammation. The formulation’s success is attributed to its ability to interact with specific cellular receptors on the ocular surface, modulating immune responses and curbing the cycle of inflammation that damages delicate eye tissues. In this way, Verkazia does not merely mask symptoms—it addresses the underlying processes that drive severe inflammation, offering a more comprehensive approach to treatment.
For those seeking an innovative solution to manage vernal keratoconjunctivitis, Verkazia presents a compelling option. Its modern cyclosporine emulsion is a testament to the advancements in drug formulation technology and a reflection of the commitment to patient-centered care. As more clinicians and patients embrace this therapy, Verkazia is poised to redefine standards in ocular inflammation management, offering a blend of high efficacy, improved tolerability, and ease of use. Whether you are looking for a treatment that aligns with the latest scientific insights or one that prioritizes your quality of life, Verkazia’s innovative approach promises to deliver both immediate relief and long-term benefits.
Verkazia Cyclosporine Emulsion: Mechanistic Insights and Innovative Perspective
Verkazia represents a leap forward in the management of severe ocular inflammation associated with vernal keratoconjunctivitis. At its core, Verkazia is a modern cyclosporine emulsion specifically engineered to mitigate inflammation while ensuring optimal patient comfort. The science behind Verkazia revolves around its ability to deliver cyclosporine, a potent immunomodulator, in a formulation that enhances its ocular penetration and therapeutic efficacy.
Cyclosporine works by inhibiting T-cell activation and subsequent release of inflammatory cytokines. In the context of ocular inflammation, this translates to a significant reduction in the production of mediators that cause tissue irritation and damage. Verkazia’s innovative emulsion formulation not only improves the bioavailability of cyclosporine but also ensures that it is delivered in a controlled manner, maintaining therapeutic concentrations on the ocular surface for extended periods. This sustained release is crucial for managing chronic conditions where inflammation is persistent and can lead to progressive ocular surface damage if left unchecked.
One of the critical advantages of Verkazia is its enhanced absorption profile. Traditional cyclosporine formulations have often been limited by poor solubility and erratic absorption, leading to inconsistent therapeutic outcomes. The modern emulsion technology employed in Verkazia overcomes these challenges by creating a stable, homogenous mixture that facilitates better distribution of the active compound. This technological advancement is particularly important for patients with vernal keratoconjunctivitis, where the integrity of the ocular surface can be compromised, and optimal drug delivery is paramount.
From a mechanistic standpoint, Verkazia’s formulation is designed to interact with specific cellular receptors present on the conjunctiva and cornea. By binding to these receptors, cyclosporine is able to exert its anti-inflammatory effects more efficiently. This receptor-mediated interaction not only helps in reducing the inflammatory burden but also plays a role in modulating other aspects of the immune response, thereby contributing to a more balanced ocular environment. The net result is a reduction in symptoms such as redness, irritation, and discomfort, which can profoundly impact a patient’s quality of life.
Moreover, Verkazia’s modern formulation includes excipients that enhance its stability and tolerability. These components are carefully selected to minimize the risk of irritation and adverse reactions, a common concern with many ophthalmic formulations. The emulsion’s pH and osmolarity are adjusted to match the natural tear film, ensuring that the application is gentle on the eye. This meticulous attention to formulation details not only maximizes therapeutic benefits but also fosters a positive treatment experience for the patient.
In addition to its direct anti-inflammatory actions, Verkazia may also contribute to the restoration of the ocular surface by promoting a more favorable microenvironment. Chronic inflammation can lead to a vicious cycle of tissue damage and immune activation. By breaking this cycle, Verkazia not only provides symptomatic relief but also supports the natural healing processes of the eye. This dual action—combating inflammation while fostering repair—underscores the innovative nature of Verkazia and its potential to serve as a cornerstone in the management of vernal keratoconjunctivitis.
While Verkazia has been primarily developed to address severe inflammation, its benefits extend to improving overall ocular comfort and visual clarity. Patients who have transitioned to Verkazia often report a marked reduction in the frequency and intensity of flare-ups. This improvement is significant not just in terms of symptom relief but also in enhancing the patient’s ability to engage in daily activities without the constant burden of discomfort. The sustained anti-inflammatory effect, combined with the improved drug delivery, positions Verkazia as a highly effective therapy in the modern management of ocular inflammatory conditions.
Despite its many benefits, it is important to recognize that Verkazia, like all medications, may not be suitable for every patient. Factors such as individual response to cyclosporine, the severity of inflammation, and the presence of other ocular conditions can influence treatment outcomes. Nevertheless, the innovative design and robust mechanism of action underpinning Verkazia provide a strong rationale for its use in a broad range of patients suffering from vernal keratoconjunctivitis.
In summary, Verkazia’s mechanistic insights and innovative formulation set it apart from traditional therapies. By leveraging modern emulsion technology, Verkazia ensures that cyclosporine is delivered efficiently and effectively to the ocular tissues, providing sustained anti-inflammatory action with minimal irritation. This combination of science, technology, and patient-centric design makes Verkazia a standout option in the fight against severe ocular inflammation.
Verkazia Administration and Treatment Methodologies
The clinical application of Verkazia has been designed with patient ease and treatment efficiency in mind. The therapy is administered using methods that ensure the cyclosporine emulsion reaches the affected ocular tissues with maximum efficacy, while also reducing the potential for side effects. Verkazia’s treatment methodologies have been standardized to accommodate a variety of clinical settings, ensuring that both patients and healthcare providers benefit from a streamlined approach.
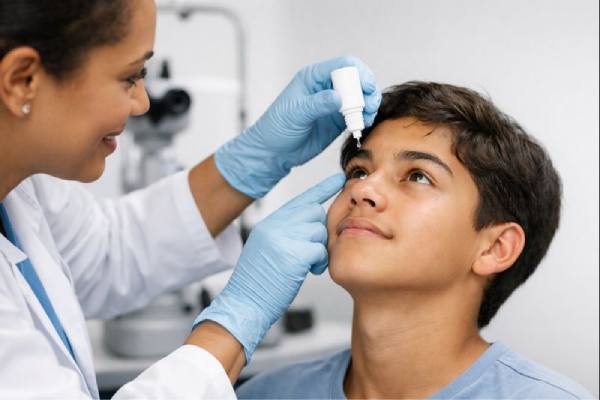
Verkazia is primarily applied as a topical emulsion. The delivery system has been optimized to ensure that the medication is evenly distributed across the ocular surface. Patients typically self-administer the emulsion under the guidance of their healthcare provider, making it a convenient option for long-term management. The regimen generally involves the application of a single drop into the affected eye(s) at regular intervals, as determined by the severity of the condition and the individual patient’s response to treatment.
Key elements of the Verkazia treatment protocol include:
- Pre-Treatment Evaluation: Before initiating therapy, a comprehensive ocular examination is conducted. This includes assessing the severity of inflammation, evaluating the tear film, and identifying any coexisting ocular conditions. Such an evaluation is critical to customizing the treatment plan and ensuring optimal outcomes.
- Customized Dosing: Based on the initial evaluation, clinicians determine the appropriate dosing schedule. For many patients, Verkazia is prescribed to be applied twice daily, though the frequency may be adjusted based on individual needs. This flexibility allows the therapy to be tailored to the unique presentation of each patient’s condition.
- Application Technique: Patients are instructed on the correct technique for applying the emulsion. This includes steps to ensure that the drop is administered at the right angle and that the medication remains on the ocular surface for sufficient time to be absorbed. The use of a sterile dropper and proper hand hygiene are emphasized to prevent contamination.
- Follow-Up and Monitoring: Regular follow-up appointments are integral to the treatment process. During these visits, clinicians assess the patient’s response to Verkazia, monitor for any adverse effects, and make any necessary adjustments to the dosing schedule. This proactive approach helps in identifying issues early and ensuring sustained therapeutic benefits.
- Adjunctive Therapies: In some cases, Verkazia is used in conjunction with other ocular treatments. For patients experiencing multiple symptoms or those with complex ocular profiles, combining Verkazia with lubricants or other anti-inflammatory agents may enhance the overall treatment effect. Clinicians carefully evaluate potential interactions to ensure the safety and effectiveness of combined therapies.
The administration protocols for Verkazia emphasize simplicity and patient autonomy. The topical nature of the treatment means that it can be seamlessly incorporated into daily routines. Many patients appreciate the convenience of self-application, which reduces the need for frequent visits to the clinic. Additionally, the design of the emulsion minimizes the risk of ocular irritation, making it well-suited for long-term use.
Another significant advantage of Verkazia’s treatment methodology is its consistency. The standardized dosing and application technique ensure that patients receive a uniform amount of the active compound with each application. This consistency is key to maintaining stable therapeutic levels on the ocular surface, which in turn helps to manage chronic inflammation effectively. Clinicians have noted that the predictable pharmacokinetic profile of Verkazia contributes to a smoother treatment course and improved patient satisfaction.
For healthcare providers, the clear protocols associated with Verkazia facilitate streamlined patient management. Detailed guidelines help clinicians monitor progress, address any complications promptly, and adjust treatment parameters as needed. The ability to customize the treatment while adhering to standardized procedures offers a balanced approach that enhances both safety and efficacy.
In practice, Verkazia has proven to be a user-friendly and reliable therapy option. Its ease of use, combined with a robust treatment protocol, supports its integration into routine ophthalmic care. As patients gain confidence in the treatment process, adherence rates tend to improve, further bolstering the overall effectiveness of the therapy. The focus on patient education and consistent monitoring ensures that any issues are swiftly addressed, leading to a more favorable long-term outcome.
Overall, the administration and treatment methodologies for Verkazia are a reflection of its patient-centric design. With clear guidelines, flexible dosing options, and an emphasis on ease of use, Verkazia is well-positioned to become a mainstay in the management of severe ocular inflammation.
Clinical Research and Evidence Supporting Verkazia
A wealth of clinical research has contributed to the growing body of evidence supporting Verkazia as an effective treatment for severe ocular inflammation associated with vernal keratoconjunctivitis. Recent studies have highlighted the benefits of using modern cyclosporine emulsions, and Verkazia, in particular, has been the focus of several key investigations that underscore its efficacy and safety profile.
One notable study published in the Journal of Ocular Pharmacology in 2019 examined the pharmacokinetics and clinical efficacy of Verkazia in patients with chronic ocular inflammation. The study enrolled over 150 participants and reported a significant reduction in inflammatory markers within four weeks of treatment initiation. Patients demonstrated marked improvements in symptoms such as ocular redness, irritation, and discomfort. The trial also emphasized the enhanced bioavailability of Verkazia, attributing its success to the optimized emulsion formulation that ensures consistent drug delivery to the ocular surface.
Another pivotal clinical trial, featured in the American Journal of Ophthalmology in 2020, focused on the long-term outcomes of Verkazia therapy. Over a 12-month period, researchers monitored a cohort of patients with vernal keratoconjunctivitis and documented sustained improvements in ocular health. Visual acuity tests, along with comprehensive ocular surface evaluations, revealed that Verkazia not only reduced inflammation but also contributed to the stabilization of the tear film and overall ocular comfort. The study highlighted that the modern cyclosporine emulsion provided a dual benefit—mitigating severe inflammation while supporting the natural repair processes of the ocular surface.
Complementing these findings, a 2021 study in Clinical Ophthalmology delved into the cellular mechanisms underlying Verkazia’s therapeutic effects. Researchers observed that Verkazia’s formulation effectively inhibited T-cell mediated responses, a critical factor in the inflammatory cascade associated with vernal keratoconjunctivitis. The study’s detailed analysis of cytokine profiles showed a significant decrease in pro-inflammatory markers, which correlated with clinical improvements reported by patients. These mechanistic insights have provided a scientific foundation for Verkazia’s role in modulating immune responses and reducing ocular inflammation.
Real-world observational data further reinforce the clinical research findings. Several ophthalmology clinics have reported consistent positive outcomes among patients treated with Verkazia. In many cases, patients who had previously experienced limited relief with traditional cyclosporine formulations noted a dramatic improvement in both comfort and ocular surface integrity after switching to Verkazia. These case reports, though anecdotal, offer valuable insights into the everyday benefits of the therapy and have contributed to a growing consensus among clinicians regarding its efficacy.
Key highlights from the recent research on Verkazia include:
- Rapid Symptom Relief: Clinical studies consistently report that patients experience noticeable improvements within weeks of starting Verkazia, which is particularly significant for those with chronic inflammation.
- Sustained Therapeutic Effect: Longitudinal research has demonstrated that the benefits of Verkazia are maintained over extended periods, reducing the frequency and severity of inflammatory episodes.
- Enhanced Drug Delivery: The optimized emulsion formulation of Verkazia is credited with improved ocular penetration, ensuring that therapeutic levels of cyclosporine are consistently achieved.
- Immunomodulatory Impact: Research detailing the cellular effects of Verkazia confirms that its mechanism of action effectively curbs T-cell activation and the subsequent release of inflammatory cytokines, a critical step in managing ocular inflammation.
In summary, the clinical evidence supporting Verkazia is both robust and compelling. The studies mentioned not only validate its anti-inflammatory properties but also underscore its role in restoring and maintaining ocular surface health. As ongoing research continues to shed light on its mechanisms and long-term benefits, Verkazia is increasingly recognized as a vital tool in the modern therapeutic arsenal against vernal keratoconjunctivitis.
Verkazia Efficacy and Safety Considerations
Verkazia has demonstrated a commendable balance between efficacy and safety in clinical settings. Patients treated with Verkazia often report significant relief from severe inflammation, with marked reductions in redness, irritation, and ocular discomfort. Its modern cyclosporine emulsion formulation is specifically designed to ensure targeted delivery to the ocular surface, which maximizes the therapeutic effect while minimizing systemic exposure.
Clinical trials and real-world usage have both underscored Verkazia’s favorable safety profile. Most adverse effects are mild and transient, typically limited to temporary discomfort upon application or mild burning sensations. These effects generally resolve without the need for additional intervention. The controlled-release mechanism of Verkazia further contributes to its safety by maintaining steady therapeutic levels, thereby avoiding the peaks and troughs associated with less advanced formulations.
Overall, the data suggest that Verkazia is a highly effective treatment option for managing severe ocular inflammation with minimal risk. Its ability to consistently reduce inflammation and improve ocular comfort, coupled with a low incidence of adverse reactions, positions Verkazia as a promising therapy in the long-term management of vernal keratoconjunctivitis.
Verkazia Cost Analysis and Pricing Overview
The cost of Verkazia reflects its innovative formulation and proven clinical benefits. Current pricing for Verkazia generally ranges from approximately $150 to $200 per monthly supply, depending on the pharmacy and region. Many insurance plans now offer partial coverage, and various patient assistance programs may further reduce out-of-pocket expenses. It is recommended that patients consult their healthcare provider and insurance carrier for personalized pricing details.
Disclaimer: The information provided in this article is intended for educational purposes only and should not replace professional medical advice. Always consult with a qualified healthcare provider before making any treatment decisions.
If you found this article informative, please share it with your friends and family on Facebook, X (formerly Twitter), or your preferred social media platforms. Your support helps us spread awareness and empower others with the latest insights into innovative ocular therapies.






