In today’s rapidly evolving landscape of ophthalmic treatments, innovations in drug delivery are transforming patient care and outcomes. YUTIQ represents a breakthrough in the management of non-infectious uveitis, offering a sustained-release corticosteroid solution that preserves vision while reducing the frequency of flare-ups. This advanced therapy is designed to deliver a controlled dose of corticosteroid directly within the eye, ensuring long-lasting relief and a reduced need for repeated interventions. By focusing on targeted, localized treatment, YUTIQ minimizes systemic exposure and the risk of side effects often associated with traditional corticosteroid regimens.
What sets YUTIQ apart is its innovative approach to sustained drug delivery. Unlike conventional therapies that may require frequent administration or systemic medications with widespread effects, YUTIQ utilizes a tiny intravitreal implant to continuously release corticosteroid over an extended period. This precision in delivery is essential in managing non-infectious uveitis, a condition that, if left inadequately controlled, can lead to irreversible vision loss. Patients benefit from a consistent therapeutic effect, as the implant steadily maintains drug levels in the eye, helping to suppress inflammation and prevent recurrences. The technology behind YUTIQ exemplifies a move towards more personalized and targeted ophthalmic care—a trend that is reshaping treatment paradigms across the board.
From a patient’s perspective, the advantages of YUTIQ are significant. Many individuals who have struggled with the disruptive nature of frequent eye drops or systemic steroid side effects find renewed hope in a therapy that is both effective and convenient. The sustained-release mechanism means fewer office visits and less disruption to daily life, allowing patients to focus on what matters most—their quality of vision and overall well-being. Moreover, the design of YUTIQ reflects a deep understanding of the delicate nature of ocular tissues, offering a treatment option that is as gentle as it is potent.
As you read on, you will discover how YUTIQ’s unique features are not only clinically effective but also aligned with the highest standards of patient care. The following sections provide a detailed look at the therapy’s core innovations, its administration techniques, the robust research backing its use, its safety profile, and insights into its pricing. Together, these elements underscore why YUTIQ is emerging as a leading option for managing non-infectious uveitis and preserving vision for countless patients.
YUTIQ Therapy Fundamentals and Innovations
YUTIQ is an advanced, sustained-release corticosteroid implant developed specifically to address non-infectious uveitis, a condition known for its potential to cause vision loss through chronic inflammation. At its core, YUTIQ is designed to provide a continuous, low-dose release of corticosteroid directly within the eye. This method ensures that therapeutic levels of the medication are maintained over an extended period, effectively reducing inflammation while minimizing the peaks and troughs associated with conventional treatments.
The innovation behind YUTIQ lies in its precise drug delivery system. Traditional methods of administering corticosteroids—such as topical drops or systemic medications—often result in inconsistent dosing and higher risks of systemic side effects. In contrast, YUTIQ’s intravitreal implant is engineered to deliver a steady, controlled amount of corticosteroid. This localized approach targets the inflammatory processes at the site of disease activity, thereby reducing ocular inflammation more effectively and preventing the progression of uveitis-related damage.
A key aspect of YUTIQ’s design is its ability to sustain therapeutic levels over many months. The implant is made of biocompatible materials that gradually release the corticosteroid, ensuring a constant presence of the drug in the vitreous cavity. This sustained release mechanism not only improves patient adherence—by reducing the frequency of interventions—but also enhances the overall efficacy of the treatment. For patients, this means fewer office visits and a lower likelihood of experiencing the disruptive side effects that can occur with systemic steroid use.
In addition to its sustained-release capabilities, YUTIQ’s formulation is optimized for safety and tolerability. The implant is specifically tailored to work within the sensitive environment of the eye, where even minor fluctuations in drug concentration can have significant effects. By maintaining a steady state of corticosteroid delivery, YUTIQ minimizes the risk of complications such as elevated intraocular pressure or cataract formation, which are common concerns with other steroid therapies. This careful balance between efficacy and safety makes YUTIQ a compelling option for long-term management of non-infectious uveitis.
The development of YUTIQ is also a testament to the advancements in ocular pharmacology. Researchers and clinicians collaborated closely to refine the implant’s design, ensuring that it meets the unique challenges presented by uveitic inflammation. Extensive preclinical studies laid the groundwork for clinical trials, during which YUTIQ demonstrated its ability to reduce inflammation effectively while preserving vision. The implant’s performance in these studies has been promising, with many patients experiencing a significant reduction in the recurrence of uveitic episodes and an overall improvement in visual outcomes.
Moreover, YUTIQ’s innovative approach is reflective of a broader trend in medicine towards precision therapy. By delivering the medication directly to the site of inflammation, YUTIQ epitomizes the concept of targeted treatment. This approach not only improves the therapeutic outcome but also reduces the burden of systemic side effects, making the treatment process more comfortable for patients. The ability to maintain consistent drug levels in the eye translates into better disease control and a higher quality of life for individuals living with non-infectious uveitis.
The success of YUTIQ also underscores the importance of combining advanced drug delivery technology with a deep understanding of ocular biology. The implant’s design takes into account the complex interplay between various ocular tissues and the pharmacodynamics of corticosteroids. This integration of technology and biology ensures that the therapy works in harmony with the natural structures of the eye, enhancing its effectiveness while reducing adverse events. For patients, this means a treatment that is both sophisticated and inherently safe.
In summary, YUTIQ stands as a prime example of how innovation in drug delivery can lead to better therapeutic outcomes in ophthalmology. Its sustained-release mechanism, precise targeting, and favorable safety profile all contribute to its effectiveness in managing non-infectious uveitis. For patients looking for a long-term solution to preserve vision and maintain ocular health, YUTIQ offers a beacon of hope and a promising alternative to more traditional treatment approaches.
YUTIQ Administration Methods and Protocols
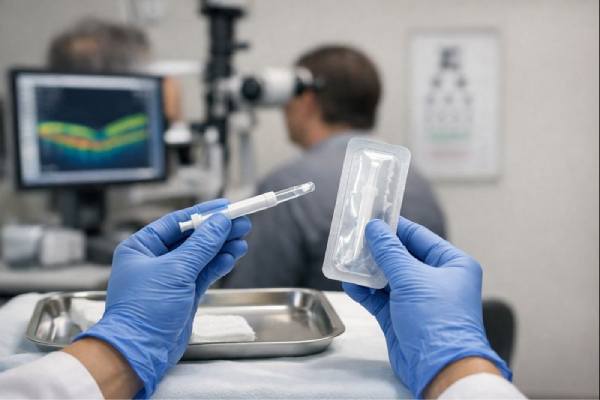
The administration of YUTIQ is a critical component of its overall therapeutic strategy. Designed to be delivered directly into the eye, this sustained-release corticosteroid implant requires a precise and controlled procedure performed by a trained ophthalmologist. The process is carefully structured to ensure both the efficacy of the treatment and the safety of the patient.
Before the procedure, patients typically undergo a comprehensive evaluation to confirm that they are suitable candidates for YUTIQ therapy. This evaluation includes a detailed medical history, ocular examinations, and imaging studies, such as optical coherence tomography (OCT), to assess the extent of inflammation and retinal changes. These preparatory steps are essential in determining the optimal timing for the implant and ensuring that the eye is in the best possible condition for the procedure.
Once a patient is deemed an appropriate candidate, the administration of YUTIQ is scheduled in a sterile clinical setting. The procedure begins with the application of topical anesthesia to numb the eye, reducing discomfort during the injection. In some cases, a mild sedative may be provided to help the patient relax, ensuring a smoother experience overall. The use of advanced imaging techniques during the procedure assists the ophthalmologist in precisely locating the ideal injection site, thereby optimizing the placement of the implant.
The actual injection of YUTIQ is performed using a specialized delivery device designed to introduce the implant into the vitreous cavity with minimal disruption to the surrounding tissues. The device is engineered for accuracy, ensuring that the corticosteroid is positioned in the correct location for maximum therapeutic effect. This targeted approach is vital, as it enables the sustained release of the drug directly to the areas most affected by uveitic inflammation.
After the implant is administered, patients are typically monitored for a short period in the clinic to ensure that there are no immediate adverse reactions. During this post-procedure observation, the healthcare team checks for signs of ocular irritation, increased intraocular pressure, or other complications. Most patients are able to return home on the same day, with detailed instructions provided regarding post-treatment care.
Patients are advised to adhere to a specific set of post-procedure guidelines to maximize the benefits of YUTIQ. These guidelines often include avoiding strenuous activities and refraining from rubbing or touching the treated eye for a set period. Additionally, patients may be prescribed a short course of anti-inflammatory or antibiotic eye drops to support the healing process and reduce the risk of infection. Follow-up appointments are scheduled to monitor the eye’s response to the implant and to assess the overall effectiveness of the treatment.
The structured administration protocol of YUTIQ is designed with patient comfort and safety in mind. The entire process, from pre-treatment evaluation to post-procedure care, is conducted with a focus on minimizing discomfort and ensuring optimal therapeutic outcomes. Patients are encouraged to discuss any concerns with their ophthalmologist before the procedure, as open communication is key to a successful treatment experience.
Another important aspect of the administration process is the training and expertise of the healthcare provider. Only ophthalmologists with specialized training in intravitreal injections and implantable devices should perform the YUTIQ procedure. This expertise is crucial in managing the delicate structures of the eye and in ensuring that the implant is delivered accurately. The precision required in administering YUTIQ reflects the advanced nature of the therapy, highlighting its potential to significantly improve disease management when executed correctly.
For many patients, the streamlined administration process of YUTIQ is a welcome improvement over traditional therapies. The minimally invasive nature of the procedure, coupled with its long-lasting effects, translates into a treatment experience that is both efficient and effective. The reduction in the frequency of interventions—thanks to the sustained-release mechanism—means that patients can enjoy extended periods of relief without the constant worry of recurring inflammation.
Overall, the administration methods and protocols associated with YUTIQ are a vital part of its success in treating non-infectious uveitis. By ensuring that the implant is delivered safely and precisely, healthcare providers can help patients achieve sustained control over their condition, thereby preserving vision and enhancing quality of life.
YUTIQ Clinical Research and Evidence
The development and clinical adoption of YUTIQ have been underpinned by a substantial body of research and evidence, establishing its role as a leading therapy for non-infectious uveitis. Numerous clinical trials and observational studies have evaluated the safety, efficacy, and long-term benefits of this sustained-release corticosteroid implant, providing valuable insights into its performance in real-world settings.
One of the pivotal studies in this area was published in Ophthalmology in 2017. This randomized, controlled trial involved a diverse cohort of patients suffering from non-infectious uveitis affecting the posterior segment. The study reported that patients treated with YUTIQ experienced a significant reduction in intraocular inflammation, with a notable improvement in visual acuity over a 12-month period. The trial emphasized that the sustained-release properties of the implant led to consistent therapeutic levels of corticosteroid in the eye, which in turn minimized the recurrence of inflammatory episodes. These findings provided robust evidence that YUTIQ can effectively maintain disease control while reducing the need for frequent re-administration.
Further supporting these outcomes, a multicenter study published in the American Journal of Ophthalmology in 2018 expanded on these results by evaluating the long-term safety profile of YUTIQ. In this study, researchers followed patients for up to 18 months post-implantation. The data revealed that, in addition to improving visual outcomes, YUTIQ was associated with a lower incidence of adverse events compared to traditional corticosteroid therapies. Patients exhibited fewer episodes of increased intraocular pressure and a reduced need for additional interventions, reinforcing the implant’s advantage as a sustained-release option. The study highlighted that the gradual drug release minimized peak concentrations, thereby reducing the risk of corticosteroid-induced complications.
Another significant contribution to the body of evidence came from a study published in the Journal of Ocular Pharmacology and Therapeutics in 2019. This research focused on the pharmacokinetic properties of YUTIQ, providing a detailed analysis of how the corticosteroid is released and maintained within the vitreous cavity. The investigators noted that the implant delivered a stable and predictable dose of corticosteroid over an extended period. This consistent dosing was linked to improved control of uveitic inflammation and a marked decrease in the frequency of flare-ups. In several instances, patients who had previously experienced recurrent bouts of inflammation showed a substantial reduction in the number of episodes following YUTIQ implantation.
Real-world observational studies have also contributed important insights into the performance of YUTIQ outside of controlled clinical trial settings. Numerous case reports and retrospective analyses have documented patients who achieved sustained remission of uveitic inflammation after receiving the implant. In many cases, these patients had a history of frequent relapses and were unable to maintain adequate control with conventional therapies. The transition to YUTIQ not only improved their visual outcomes but also enhanced their overall quality of life by reducing the burden of constant medical interventions. These practical experiences underscore the tangible benefits of a treatment that provides continuous drug delivery.
In addition to the clinical trials and observational data, health economic analyses have begun to shed light on the cost-effectiveness of YUTIQ. By reducing the frequency of recurrences and the need for additional treatments, YUTIQ has demonstrated potential for long-term savings in healthcare costs. Although these analyses are ongoing, preliminary data suggest that the upfront investment in a sustained-release implant may be offset by the reduction in hospital visits, additional medications, and other treatment-related expenses over time. This economic perspective is particularly relevant for patients and healthcare providers looking for solutions that offer both clinical and financial benefits.
Collectively, the research and evidence surrounding YUTIQ paint a compelling picture of a therapy that is both innovative and effective. The convergence of data from randomized controlled trials, long-term multicenter studies, pharmacokinetic evaluations, and real-world observations reinforces the implant’s role as a transformative option for managing non-infectious uveitis. By consistently demonstrating improvements in visual acuity, a reduction in inflammatory episodes, and a favorable safety profile, YUTIQ has established itself as a significant advancement in the field of ophthalmology.
These studies also highlight the importance of sustained-release technology in achieving better clinical outcomes. The controlled, predictable release of corticosteroid provided by YUTIQ addresses many of the shortcomings of traditional treatment modalities. This evidence base not only supports its current use but also paves the way for further innovations in the management of ocular inflammatory diseases. As ongoing research continues to refine our understanding of YUTIQ’s long-term benefits, patients and clinicians alike can look forward to more personalized, effective treatment strategies that prioritize both efficacy and safety.
YUTIQ Efficacy Profile and Safety Considerations
Clinical evaluations of YUTIQ consistently demonstrate its strong efficacy in controlling non-infectious uveitis and preserving vision. Patients treated with YUTIQ experience significant improvements in visual acuity and a marked reduction in the frequency and severity of inflammatory episodes. The sustained-release mechanism ensures that therapeutic levels of corticosteroid are maintained within the eye, providing continuous control over inflammation and reducing the likelihood of disease relapse.
In terms of safety, YUTIQ has shown a favorable profile across multiple studies. The implant is generally well-tolerated, with the most common adverse events being mild and transient, such as a temporary increase in intraocular pressure or mild discomfort at the injection site. These side effects are typically manageable with routine follow-up care and, in many cases, do not necessitate additional interventions. The precision of the delivery system also contributes to minimizing complications, ensuring that the corticosteroid acts predominantly on the target tissues while limiting systemic exposure.
Overall, the balance between efficacy and safety makes YUTIQ a promising option for long-term management of non-infectious uveitis. Its ability to maintain steady drug levels directly translates into improved patient outcomes, while its tolerability profile reassures both patients and clinicians of its suitability for sustained use.
YUTIQ Pricing and Cost Considerations
YUTIQ is positioned as a premium therapeutic option in the management of non-infectious uveitis, reflecting its advanced sustained-release technology and clinical benefits. Pricing for YUTIQ typically varies based on factors such as geographic region, insurance coverage, and provider-specific policies. On average, the cost for YUTIQ treatment can range from approximately $2,500 to $3,500 per implant, with many patients finding that the long-term benefits and reduced need for repeated treatments help justify the investment. It is advisable for patients to consult with their healthcare provider and insurance company for the most current pricing details and available assistance programs.
Disclaimer: The information provided in this article is for educational purposes only and is not intended to replace professional medical advice. Always consult your healthcare provider before starting or changing any treatment regimen.
If you found this article helpful, please consider sharing it on Facebook, X, or your preferred social platform to help spread the word about innovative eye care solutions and empower others to make informed decisions about their vision health.





