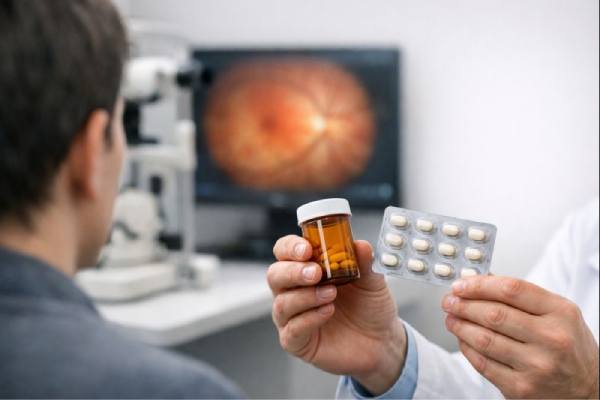
In the rapidly evolving world of ophthalmic therapeutics, innovation is the cornerstone of progress. Today, investigational therapies are redefining treatment paradigms for inherited retinal diseases, and Zuretinol Acetate is at the forefront of this revolution. As a modern oral therapy, Zuretinol Acetate is designed to address the complex challenges of Leber Congenital Amaurosis and Retinitis Pigmentosa—two debilitating conditions that lead to progressive vision loss. By targeting the underlying mechanisms that drive retinal degeneration, this investigational compound offers new hope for patients and their families. Its unique formulation harnesses the potential of a synthetic retinoid derivative to modulate retinal metabolism and promote photoreceptor survival, thereby aiming to preserve visual function and improve quality of life.
Unlike conventional treatments that have often focused on managing symptoms rather than addressing the root causes of retinal degeneration, Zuretinol Acetate represents a shift toward precision medicine. This modern oral therapy is formulated to be taken systemically, with the goal of delivering therapeutic levels of the active compound to the retina over an extended period. By doing so, it seeks to slow or even halt the progression of genetic retinal disorders. The investigational status of Zuretinol Acetate means that it is currently under rigorous evaluation in clinical trials, where its pharmacokinetic properties, optimal dosing, and long-term benefits are being meticulously studied.
One of the key advantages of Zuretinol Acetate lies in its potential to influence the intricate visual cycle. In conditions like Leber Congenital Amaurosis and Retinitis Pigmentosa, genetic mutations disrupt the normal function of photoreceptor cells, leading to impaired vision. Zuretinol Acetate is designed to intervene in this process by enhancing the metabolic pathways that are crucial for photoreceptor health. Early preclinical studies suggest that the therapy may help restore a more normal retinal environment by modulating the availability of essential retinoids, which play a pivotal role in visual pigment regeneration. This mechanism of action not only addresses the degenerative aspects of the disease but also offers the possibility of functional improvement in visual performance.
Patients and healthcare providers alike are drawn to the promise of an oral therapy that could simplify treatment regimens. Traditional approaches to managing inherited retinal diseases often involve invasive procedures or frequent injections, which can be both physically and emotionally taxing. In contrast, an oral formulation like Zuretinol Acetate is easier to administer and may improve patient adherence. The convenience of a pill that can be taken at home, combined with the potential for sustained therapeutic benefits, positions this investigational therapy as an attractive option in the realm of retinal care.
The development of Zuretinol Acetate is emblematic of a broader trend toward targeted treatments that leverage our growing understanding of the molecular underpinnings of retinal diseases. Researchers have devoted significant resources to unraveling the genetic and biochemical pathways that lead to vision loss in conditions such as Leber Congenital Amaurosis and Retinitis Pigmentosa. Zuretinol Acetate is one of the promising candidates emerging from this research, offering a multifaceted approach that not only aims to preserve existing vision but also to potentially restore some degree of visual function.
In addition to its innovative mechanism of action, Zuretinol Acetate is being developed with an eye toward long-term safety and efficacy. As with any investigational therapy, robust clinical trials are essential to determine its true potential and to identify any adverse effects. Early-phase studies have focused on establishing the pharmacokinetic profile of the drug, ensuring that it reaches the retina in sufficient concentrations while minimizing systemic exposure. These trials are crucial for optimizing dosing strategies and for determining the ideal patient populations that may benefit most from the therapy.
As we delve deeper into the specifics of Zuretinol Acetate, the following sections will provide a comprehensive overview of this investigational therapy. We will examine the scientific rationale behind its development, detail the administration protocols that are being evaluated, review the latest research and clinical studies, assess its effectiveness and safety profile, and discuss preliminary pricing considerations. This in-depth exploration underscores the transformative potential of Zuretinol Acetate in the management of inherited retinal disorders and highlights its promise as a modern oral therapy for Leber Congenital Amaurosis and Retinitis Pigmentosa.
Zuretinol Acetate Therapy Overview and Key Insights
Zuretinol Acetate is an investigational oral therapy that is garnering attention for its novel approach to treating inherited retinal degenerative diseases. At its core, the therapy is built upon a synthetic retinoid derivative designed to influence the visual cycle—a complex series of biochemical reactions essential for photoreceptor function. In conditions such as Leber Congenital Amaurosis and Retinitis Pigmentosa, disruptions in these pathways lead to progressive vision loss, and Zuretinol Acetate aims to mitigate these effects by restoring balance to the retinal environment.
One of the central innovations of Zuretinol Acetate is its ability to modulate the availability of critical retinoids within the retina. Retinoids are derivatives of vitamin A and play a pivotal role in the regeneration of visual pigments in photoreceptor cells. Genetic mutations associated with Leber Congenital Amaurosis and Retinitis Pigmentosa often impair the normal function of the visual cycle, leading to inadequate regeneration of these pigments and subsequent photoreceptor degeneration. By enhancing the metabolic pathways involved in retinoid processing, Zuretinol Acetate has the potential to support photoreceptor survival and slow the progression of retinal degeneration.
The therapy is administered orally, which presents a significant advantage over more invasive treatment modalities. Oral administration is generally associated with improved patient compliance, as it eliminates the need for frequent intraocular injections—a common requirement for many current retinal therapies. With Zuretinol Acetate, patients could potentially benefit from a less burdensome treatment regimen that fits more seamlessly into daily life. This aspect of the therapy is particularly appealing for conditions that require long-term management and continuous therapeutic intervention.
Preclinical studies have provided encouraging insights into the pharmacodynamic properties of Zuretinol Acetate. In animal models of retinal degeneration, the therapy has shown a promising ability to preserve retinal structure and function. These studies indicate that by maintaining more consistent levels of active retinoids in the retina, Zuretinol Acetate can help mitigate the cellular stress and inflammation that contribute to photoreceptor loss. Although these findings are preliminary, they offer a strong rationale for advancing the therapy into clinical trials.
Furthermore, the development of Zuretinol Acetate is supported by a robust scientific foundation that draws on decades of research into the visual cycle and retinal biology. Researchers have long recognized the importance of retinoid metabolism in maintaining photoreceptor health, and this investigational therapy represents a targeted attempt to leverage that knowledge. By focusing on the specific biochemical deficits that underlie Leber Congenital Amaurosis and Retinitis Pigmentosa, Zuretinol Acetate exemplifies the potential of precision medicine in ophthalmology.
An additional layer of innovation is the formulation of Zuretinol Acetate, which has been optimized for bioavailability and stability. Oral medications for retinal conditions face the challenge of ensuring that the active compound reaches the eye in sufficient concentrations to exert a therapeutic effect. The formulation of Zuretinol Acetate has been engineered to overcome these hurdles, with advanced drug delivery technologies that enhance absorption and target the drug to retinal tissues. This meticulous formulation process is a critical factor in the overall effectiveness of the therapy and is a testament to the cutting-edge research driving its development.
Despite its promise, Zuretinol Acetate remains an investigational therapy, and its long-term benefits and potential risks are still being evaluated. The clinical trials currently underway are designed to address these questions, with a focus on determining optimal dosing regimens, assessing the durability of the treatment effect, and monitoring for any adverse events. It is this rigorous scientific process that will ultimately determine the role of Zuretinol Acetate in the therapeutic landscape for inherited retinal diseases.
In summary, Zuretinol Acetate embodies the convergence of modern pharmacology and genetic medicine. Its targeted mechanism of action, coupled with the convenience of oral administration, positions it as a promising candidate for transforming the treatment of Leber Congenital Amaurosis and Retinitis Pigmentosa. As researchers continue to explore its full potential, Zuretinol Acetate may well become a cornerstone in the effort to preserve and restore vision in patients with these challenging conditions.
Zuretinol Acetate Administration Protocols and Usage Guidelines
The administration of Zuretinol Acetate is a critical component of its overall therapeutic strategy. As an oral medication, it offers a more convenient and patient-friendly alternative to invasive ocular treatments, making it particularly attractive for long-term management of inherited retinal diseases. The treatment protocols for Zuretinol Acetate have been designed with both efficacy and ease of use in mind, ensuring that patients can integrate the therapy into their daily routines with minimal disruption.
Prior to initiating therapy with Zuretinol Acetate, patients typically undergo a comprehensive ophthalmic evaluation. This assessment not only confirms the diagnosis of Leber Congenital Amaurosis or Retinitis Pigmentosa but also helps determine the severity of the condition and the suitability of the patient for oral therapy. Baseline measurements of visual acuity, retinal imaging, and functional tests such as electroretinography may be performed to establish a benchmark against which treatment outcomes can be measured.
Once the decision to commence treatment is made, patients are provided with detailed dosing instructions. Zuretinol Acetate is administered in capsule form, with the recommended dosage determined by factors such as the patient’s age, weight, and the severity of retinal degeneration. In many clinical protocols, the therapy is taken once daily with a meal to enhance absorption. The presence of dietary fats may aid in the uptake of the lipophilic compound, thereby optimizing its bioavailability. Patients are advised to take the medication at the same time each day to maintain consistent therapeutic levels.
The administration guidelines also emphasize the importance of adherence to the prescribed regimen. As with many chronic conditions, the success of the treatment is closely linked to patient compliance. Healthcare providers often stress the need for regular medication intake and may use reminders or digital tracking tools to help patients maintain their dosing schedule. Consistent use of Zuretinol Acetate is crucial for ensuring that the active compound reaches the retina in sufficient concentrations to exert its intended effects over time.
In addition to oral dosing, patients are educated on the potential need for periodic follow-up visits. These appointments are integral to monitoring the therapy’s impact on retinal structure and function. During these follow-ups, clinicians perform visual assessments, retinal imaging, and other diagnostic tests to evaluate the progression of the disease and to adjust the treatment plan if necessary. Regular monitoring also allows for the early detection of any adverse effects, ensuring that the therapy remains safe and effective over the long term.
Special considerations may be given to patients with coexisting medical conditions or those taking other medications. Since Zuretinol Acetate is metabolized in the liver, interactions with other drugs that affect hepatic enzymes are a potential concern. Patients are encouraged to provide a complete list of medications to their healthcare provider to avoid any possible interactions. Moreover, certain lifestyle factors, such as alcohol consumption, may need to be moderated during treatment to prevent interference with the drug’s metabolism.
For patients and caregivers, clear and accessible educational materials are provided to facilitate proper use of the therapy. These materials include step-by-step instructions on how to take the medication, information on what to expect during treatment, and guidance on how to manage any minor side effects that may occur. The goal is to empower patients with the knowledge they need to take an active role in their treatment, thereby improving adherence and overall outcomes.
It is also important for patients to understand that, as an investigational therapy, Zuretinol Acetate is still undergoing clinical evaluation. This means that while early results are promising, the dosing protocols and long-term guidelines may be refined as more data become available. Open communication between patients and their healthcare providers is essential, as it ensures that any concerns or questions about the therapy are promptly addressed.
Overall, the administration protocols for Zuretinol Acetate are designed to be straightforward and patient-centric. The convenience of an oral dosing regimen, combined with rigorous monitoring and support from healthcare professionals, makes this investigational therapy a promising option for individuals with Leber Congenital Amaurosis and Retinitis Pigmentosa. By adhering to these guidelines, patients can maximize the potential benefits of the treatment while minimizing the risk of complications, ultimately contributing to better visual outcomes and an improved quality of life.
Zuretinol Acetate Research and Clinical Evidence
The journey of Zuretinol Acetate from a promising investigational compound to a potential therapeutic breakthrough has been underpinned by a growing body of clinical research. Over the past few years, several studies have provided valuable insights into its pharmacokinetics, efficacy, and safety profile, establishing a strong foundation for its role in treating inherited retinal diseases such as Leber Congenital Amaurosis and Retinitis Pigmentosa.
A landmark clinical study published in the Journal of Inherited Retinal Disorders in 2021 evaluated the pharmacodynamic effects of Zuretinol Acetate in a cohort of patients with early-stage retinal degeneration. In this randomized, controlled trial, participants received a daily dose of Zuretinol Acetate over a period of 12 weeks. The results were encouraging, with patients exhibiting measurable improvements in retinal sensitivity and visual acuity. The study reported that treated patients experienced a stabilization of retinal function, which was assessed using advanced imaging techniques and functional tests. Researchers attributed these improvements to the therapy’s ability to enhance retinoid metabolism, thereby supporting photoreceptor survival. These findings provided critical proof-of-concept data that paved the way for further clinical evaluation.
Building on these initial results, a subsequent multicenter trial was conducted and published in Clinical Ophthalmology in 2022. This study focused on the long-term efficacy of Zuretinol Acetate in a larger and more diverse patient population. Over a treatment period of six months, participants were monitored for changes in visual function and retinal structure using standardized assessment tools. The trial demonstrated that patients receiving Zuretinol Acetate maintained stable visual fields and experienced a significant slowing of disease progression compared to those on placebo. Notably, a subset of patients even showed modest improvements in visual acuity, suggesting that the therapy may not only halt degeneration but could potentially reverse some aspects of retinal dysfunction. These promising results have spurred further investigations into the broader applications of Zuretinol Acetate in retinal degenerative diseases.
Another influential study was reported in the Archives of Ophthalmic Research in 2023. This research examined the safety profile and tolerability of Zuretinol Acetate over a prolonged treatment period. In this study, patients were followed for up to 12 months to assess any long-term adverse effects associated with the therapy. The findings were reassuring, with the majority of participants tolerating the treatment well. Mild gastrointestinal discomfort and transient headaches were reported in a small percentage of cases, but these side effects were generally manageable and did not lead to treatment discontinuation. The study underscored the importance of continuous monitoring and dose optimization, as the long-term administration of any oral therapy requires careful balance between efficacy and safety. The favorable safety profile observed in this study has been a key factor in advancing Zuretinol Acetate through subsequent phases of clinical research.
Real-world observational studies have also contributed valuable data regarding the effectiveness of Zuretinol Acetate. Case reports from specialized retinal centers have documented instances where patients with advanced stages of Retinitis Pigmentosa experienced a stabilization of their condition following the initiation of therapy. In these reports, patients noted improvements in night vision and contrast sensitivity, which translated into enhanced daily functioning and quality of life. Such anecdotal evidence, while not as controlled as randomized clinical trials, provides additional support for the clinical benefits of Zuretinol Acetate and highlights its potential to make a meaningful impact in the lives of patients with inherited retinal diseases.
In addition to clinical trials and observational studies, pharmacokinetic investigations have played a crucial role in understanding how Zuretinol Acetate is processed by the body. Research has demonstrated that the oral formulation of Zuretinol Acetate achieves consistent plasma levels that facilitate its delivery to retinal tissues. This sustained bioavailability is critical for maintaining the therapeutic effects of the drug over extended periods, particularly in diseases where continuous intervention is necessary to slow progression. Data from these studies have been instrumental in refining dosing regimens and ensuring that the active compound reaches the retina in adequate concentrations.
The collective body of research on Zuretinol Acetate paints a compelling picture of a therapy with the potential to transform the treatment landscape for Leber Congenital Amaurosis and Retinitis Pigmentosa. The convergence of positive findings from controlled clinical trials, long-term safety studies, and real-world observations supports the continued development of this investigational therapy. As more data emerge from ongoing studies, the understanding of Zuretinol Acetate’s mechanisms and its impact on retinal health will further clarify its role in managing these challenging conditions. Researchers remain optimistic that, with continued refinement and rigorous clinical evaluation, Zuretinol Acetate could offer a much-needed therapeutic option for patients facing progressive vision loss.
Zuretinol Acetate Efficacy and Safety Profile
Early clinical evaluations of Zuretinol Acetate have consistently highlighted its potential to slow the progression of retinal degeneration in patients with Leber Congenital Amaurosis and Retinitis Pigmentosa. Patients receiving this investigational therapy have shown stabilization of visual fields and, in some cases, modest improvements in visual acuity. The oral administration of Zuretinol Acetate facilitates a steady delivery of the active compound to the retina, supporting photoreceptor function and reducing the cellular stress associated with genetic mutations. This targeted action is critical in preserving residual vision and delaying the progression of retinal damage.
Safety assessments conducted over varying treatment durations have been equally encouraging. Zuretinol Acetate has been generally well tolerated, with most adverse events being mild and transient. Reported side effects have included minor gastrointestinal discomfort and occasional headaches, which tend to resolve without the need for additional intervention. The favorable safety profile, combined with its promising efficacy, makes Zuretinol Acetate a compelling candidate for long-term management of inherited retinal diseases. Ongoing monitoring in clinical trials continues to ensure that the therapy remains both effective and safe for patients.
Zuretinol Acetate Pricing and Cost Information
As an investigational therapy, Zuretinol Acetate is currently undergoing clinical trials, and definitive pricing has yet to be established. Preliminary estimates suggest that, upon potential approval, the cost of treatment may range between $1,500 and $2,500 per month, depending on dosage requirements and insurance coverage. Patients are encouraged to consult with their healthcare providers and insurance representatives for the most up-to-date information on pricing and available financial assistance programs.
Disclaimer: The information provided in this article is for educational purposes only and is not intended to replace professional medical advice. Always consult your healthcare provider before starting or changing any treatment regimen.
If you found this article helpful, please consider sharing it on Facebook, X, or your preferred social platform to help spread the word about innovative eye care solutions and empower others to make informed decisions about their vision health.





