What is phacomatoses?
Phacomatoses, also known as neurocutaneous syndromes, are a group of hereditary disorders distinguished by the presence of lesions on both the skin and the nervous system. These conditions are usually genetic, with abnormalities in the development and function of multiple tissues such as the eyes, brain, spinal cord, and skin. The term “phacomatosis” comes from the Greek word “phakos,” which means “birthmark,” referring to the common occurrence of skin lesions in these disorders. The most well-known phacomatoses are: Neurofibromatosis Type 1 (NF1), Neurofibromatosis Type 2 (NF2), Tuberous Sclerosis Complex (TSC), Sturge-Weber Syndrome (SWS), and Von Hippel-Lindau Disease (VHL).
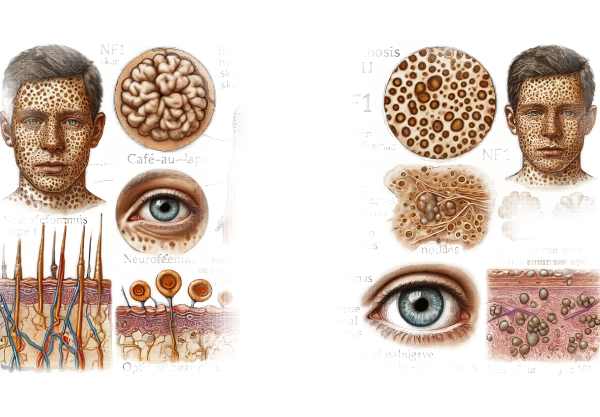
Neurofibromatosis Type I (NF1)
Overview: NF1, or von Recklinghausen disease, is the most common phacomatosis. Mutations in the NF1 gene, which encodes neurofibromin, a protein that regulates cell growth, cause this autosomal dominant disorder.
Symptoms: Multiple café-au-lait spots (light brown skin patches), neurofibromas (benign nerve sheath tumors), Lisch nodules (benign iris hamartomas), and freckling in the axillary and inguinal regions are the hallmarks of NF1. Affected people may also develop skeletal abnormalities, learning disabilities, and a higher risk of certain cancers, such as optic pathway gliomas and malignant peripheral nerve sheath tumors.
Neurofibromatosis Type 2 (NF)
Overview: NF2 is a less common but more severe form of neurofibromatosis that is also autosomal dominant. Mutations in the NF2 gene, which encodes the tumor suppressor protein merlin (schwannomin), are the cause.
Symptoms: The primary feature of NF2 is the development of bilateral vestibular schwannomas (acoustic neuromas), which cause hearing loss, tinnitus, and balance problems. Other characteristics include meningiomas, ependymomas, and cataracts. Skin manifestations are less noticeable than in NF1, but can include schwannomas and café-au-lait spots.
Tuberous Sclerosis Complex(TSC)
Overview: TSC is an autosomal dominant disorder caused by mutations in either the TSC1 or TSC2 genes, which produce hamartin and tuberin, respectively. These proteins form a complex that controls cell growth and differentiation.
Symptoms: TSC is characterized by the development of benign tumors known as hamartomas in various organs, including the brain, heart, kidneys, lungs, and skin. Common skin manifestations are hypomelanotic macules (white patches), facial angiofibromas, shagreen patches (thickened, leathery skin), and periungual fibromas. Neurological manifestations include epilepsy, intellectual disability, and autism spectrum disorder.
Sturge–Weber Syndrome (SWS)
Overview: Somatic mutations in the GNAQ gene cause SWS, also known as encephalotrigeminal angiomatosis. It is defined by vascular abnormalities in the brain, skin, and eyes.
Symptoms: The most distinguishing feature of SWS is a facial port-wine stain (capillary malformation) that usually follows the trigeminal nerve distribution. Seizures, developmental delay, and hemiparesis are all possible neurological complications. Glaucoma and choroidal hemangiomas are two examples of ocular manifestations that can cause blindness.
Von Hippel–Lindau Disease (VHL)
Overview: Mutations in the VHL gene, which encodes a protein involved in the regulation of hypoxia-inducible factors, cause VHL, an autosomal dominant disorder.
Symptoms: VHL causes the development of a variety of benign and malignant tumors, including brain and retinal hemangioblastomas, renal cell carcinoma, pheochromocytomas, and pancreatic neuroendocrine tumors. Retinal hemangioblastomas can cause vision loss if not treated.
Pathophysiology
Phacomatoses’ pathophysiology is characterized by genetic mutations that cause dysregulation of cellular growth and differentiation. These mutations cause the formation of hamartomas, benign and malignant tumors, and other abnormalities that affect multiple organ systems. Each phacomatosis is associated with a specific genetic mutation and pathway:
- NF1: A mutation in the NF1 gene causes neurofibromin to lose function, which normally inhibits the RAS/MAPK signaling pathway, resulting in uncontrolled cell proliferation.
- NF2: The NF2 gene mutation causes the loss of merlin function, which is a protein that regulates cell growth and contact inhibition.
- TSC: Mutations in TSC1 or TSC2 disrupt the TSC1-TSC2 complex, activating the mTOR pathway and promoting cell growth and proliferation.
- SWS: Somatic mutations in the GNAQ gene result in abnormal vascular and capillary malformations.
- VHL: Mutations in the VHL gene alter the degradation of hypoxia-inducible factors, resulting in abnormal vascular and tumor growth.
Genetic Inheritance
Most phacomatoses have an autosomal dominant inheritance pattern, which means that a single copy of the mutated gene is enough to cause the disorder. Even among individuals with the same mutation, the severity and expression of symptoms vary significantly. Some phacomatoses, such as SWS, are sporadic and do not have a predictable inheritance pattern.
Diagnostic methods
Dermatologists, neurologists, ophthalmologists, geneticists, and other specialists must all collaborate to diagnose phacomatoses. Here are the main diagnostic methods:
Clinical Examination
A thorough clinical examination is essential for diagnosing phacomatoses. This includes:
Skin Examination: Dermatological examination for common lesions such as café-au-lait spots, neurofibromas, hypomelanotic macules, angiofibromas, port-wine stains, and periungual fibromas. The distribution, size, and number of these lesions can provide useful diagnostic information.
Neurological Examination: Evaluation of neurological function, including seizure detection, developmental delays, motor and sensory deficits, and cranial nerve abnormalities. Detailed history-taking is essential for documenting the onset and progression of neurological symptoms.
Ophthalmologic Examination: A thorough eye exam to detect ocular manifestations like Lisch nodules, retinal hemangioblastomas, optic pathway gliomas, and glaucoma. Slit lamp examination, fundoscopy, and intraocular pressure measurement are some of the techniques used.
Imaging Studies
Imaging studies play an important role in diagnosing and monitoring phacomatoses.
Magnetic Resonance Imaging (MRI): MRI is the preferred imaging technique for examining the brain and spinal cord. It is capable of detecting tumors, structural abnormalities, and other lesions associated with phacomatoses. MRI with contrast enhancement is especially effective in detecting meningiomas, schwannomas, and hemangioblastomas.
Computed Tomography (CT) Scan: CT scans can detect calcifications, bone abnormalities, and some types of tumors. They are frequently used in conjunction with MRI to provide a thorough evaluation.
Ultrasound: Abdominal ultrasound is used to detect renal and pancreatic tumors, particularly in cases of VHL. Ultrasound is also useful in detecting cardiac rhabdomyomas in TSC.
Ophthalmologic Imaging: Techniques like optical coherence tomography (OCT) and fluorescein angiography are used to evaluate retinal and choroidal lesions. These imaging modalities provide detailed views of ocular structures, assisting in the early detection and monitoring of eye-related complications.
Treatment Options for Phacomatoses
The treatment for phacomatoses is multifaceted, with the goal of managing symptoms, preventing complications, and improving overall quality of life. Given the complexities and variability of these conditions, a collaborative approach involving dermatologists, neurologists, ophthalmologists, geneticists, and other specialists is required. The following are the main treatment strategies for some of the primary phacomatoses:
Neurofibromatosis Type I (NF1)
Surgical Removal of Neurofibromas: Surgical removal is an option for neurofibromas that are symptomatic or cosmetically significant. Surgery is generally reserved for tumors that cause pain, functional impairment, or disfigurement.
Management of Optic Pathway Gliomas: These tumors, which are common in NF1, are typically monitored using regular imaging. If the tumor grows or causes vision problems, chemotherapy or radiation treatment may be required.
Learning and Behavioral Support: Children with NF1 frequently require educational assistance, speech therapy, and behavioral interventions to address learning disabilities and attention deficits.
Neurofibromatosis Type 2 (NF)
Surgical Removal of Vestibular Schwannomas: These tumors can cause significant morbidity, including hearing loss and balance issues. These tumors may be treated surgically or with stereotactic radiosurgery.
Hearing Rehabilitation: Patients with NF2 may benefit from hearing aids, cochlear implants, or auditory brainstem implants to manage hearing loss.
Management of Meningiomas and Ependymomas: Depending on their size, location, and rate of growth, these tumors may necessitate surgery, radiation therapy, or close monitoring.
Tuberous Sclerosis Complex(TSC)
Antiepileptic Drugs (AEDs): Seizures are common in TSC and are usually treated with AEDs. Specific medications, such as vigabatrin, may be especially effective for infantile spasms caused by TSC.
mTOR Inhibitors: Drugs like everolimus and sirolimus can shrink renal angiomyolipomas, cardiac rhabdomyomas, and subependymal giant cell astrocytomas by inhibiting the mTOR pathway.
Behavioral and Developmental Support: Children with TSC frequently benefit from early intervention services, such as physical, occupational, and speech therapy, as well as support for autism spectrum disorders.
Sturge–Weber Syndrome (SWS)
Laser Therapy for Port-Wine Stains: Pulsed dye laser therapy can help reduce the appearance of facial port-wine stains, which are characteristic of SWS.
Seizures in SWS are treated with antiepileptic drugs (AEDs). In refractory cases, surgical procedures such as hemispherectomy may be considered.
Glaucoma Management: Glaucoma caused by SWS is treated with medications that lower intraocular pressure, and surgical procedures may be required if medical therapy is ineffective.
Von Hippel–Lindau Disease (VHL)
Surgical Tumor Removal: Surgical resection is commonly used to treat hemangioblastomas of the brain and spinal cord, as well as renal cell carcinoma. Regular monitoring and early intervention are critical for preventing complications.
Laser and Cryotherapy for Retinal Hemangioblastomas: These treatments can help manage retinal hemangioblastomas while maintaining vision.
Monitoring and Surveillance: Regular imaging studies and blood tests are critical for the early detection and treatment of VHL-associated tumors.
Frequently Asked Questions about Phacomatoses
What Causes Phacomatoses?
Genetic mutations cause phacomatoses, which affect the development and function of a variety of tissues such as the skin, nervous system, and eyes. These conditions are usually inherited in an autosomal dominant pattern, though some may occur sporadically.
How Are Phacomatoses Diagnosed?
Clinical evaluation, imaging studies, and genetic testing are all used to make the diagnosis. MRI, CT scans, ophthalmologic examinations, and molecular genetic testing are important diagnostic tools for identifying specific mutations.
Are phacomatoses curable?
Although phacomatoses are incurable, appropriate treatment can alleviate many symptoms and complications. Early diagnosis and a multidisciplinary approach to care are critical for improving outcomes.
What are the most common symptoms of phacomatoses?
Skin lesions, neurological abnormalities, and ocular manifestations are common symptoms, though they vary by condition. Examples include café-au-lait spots and neurofibromas in NF1, seizures in TSC, and port-wine stains in SWS.
Do phacomatoses affect vision?
Yes, several phacomatoses can impair vision. For example, NF1 can cause optic pathway gliomas, TSC can cause retinal hamartomas, and VHL can result in retinal hemangioblastomas. Regular eye exams are critical for early detection and treatment.
How are seizures treated in phacomatoses?
Antiepileptic drugs (AEDs) help to treat seizures. The type of seizures and underlying phacomatosis may influence the choice of medication. In some cases, surgical interventions may be required for refractory seizures.
What types of genetic tests are available for phacomatoses?
Molecular genetic testing, including next-generation sequencing (NGS) panels, can detect specific mutations linked to phacomatoses. Affected individuals and families should also seek genetic counseling.
Are there support groups for people with phacomatoses?
Yes, several organizations offer support and resources to people with phacomatoses and their families. These groups provide educational materials, emotional support, and opportunities to connect with others who face similar challenges.
How can I deal with the emotional and social consequences of phacomatoses?
Managing the emotional and social impact entails seeking help from healthcare providers, counselors, and support groups. Early intervention, educational support, and therapy can also help people deal with the difficulties associated with these conditions.
What should I do if I suspect that my child has phacomatosis?
If you suspect your child has phacomatosis, contact a genetics specialist. Early detection and intervention are critical for controlling symptoms and avoiding complications.
Trusted Resources and Support
Books and Organizations
Books:
- “Neurocutaneous Disorders: Phakomatoses and Hamartoneoplastic Syndromes”* by Martino Ruggieri and Ignacio Pascual-Castroviejo.
- “Neurofibromatosis: A Handbook for Patients, Families, and Health Care Professionals” by Bruce Korf.
Organizations:
- Children’s Tumor Foundation (CTF): Funds neurofibromatosis research and offers assistance to patients and families.
- Tuberous Sclerosis Alliance (TSA): Provides resources, support, and advocacy to people with tuberous sclerosis complex.
- Von Hippel-Lindau Alliance (VHLA): Provides information and support to people living with Von Hippel-Lindau disease.
Financial Aid Options
Insurance Coverage: Most health insurance policies cover the diagnosis and treatment of phacomatosis. Patients should consult with their insurance companies to understand the specifics of their coverage, such as co-pays and deductibles.
Patient Assistance Programs: Pharmaceutical companies and non-profit organizations frequently provide patient assistance programs to help with the cost of medications and treatments. Programs such as RxAssist provide information about available resources.
Non-Profit Organizations: The HealthWell Foundation and the Patient Advocate Foundation provide financial assistance to patients for medical expenses, including treatments for phacomatoses.
Government Programs: Medicaid, Medicare, and the Children’s Health Insurance Program (CHIP) provide coverage to eligible individuals. These programs can help cover the costs of phacomatosis treatment and care, ensuring that patients receive the medical attention they require regardless of their financial situation.