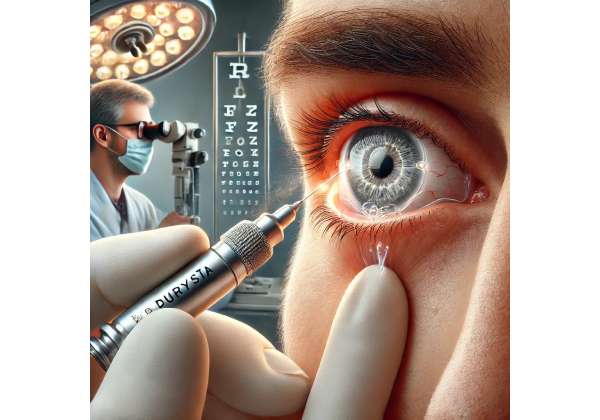
Glaucoma is a complex eye disorder and a major cause of preventable vision loss worldwide. When left unchecked, this condition gradually damages the optic nerve, often without symptoms until vision is already severely compromised. Traditional treatments, such as daily eye drops, can effectively control intraocular pressure (IOP) but often depend on strict adherence. Durysta, a bimatoprost implant, offers a long-term solution by releasing medication continuously for up to six months in the anterior chamber of the eye. This innovative approach addresses key challenges like poor compliance and fluctuating drug levels, ultimately contributing to more stable IOP management and improved outcomes for many patients.
1. Understanding Glaucoma and What Durysta Brings to the Table
Glaucoma Basics
Glaucoma encompasses a range of conditions typically linked to elevated IOP. The most common form, primary open-angle glaucoma (POAG), arises when the eye’s drainage structures—particularly the trabecular meshwork—fail to efficiently clear aqueous humor. Over time, higher pressure harms the optic nerve, leading to peripheral vision loss and possible blindness. Risk factors include older age, African or Hispanic heritage, family history, and certain medical conditions like diabetes or hypertension. Because glaucoma often proceeds silently, routine eye exams are vital for early detection.
Consequences of Elevated IOP
Unchecked IOP is widely accepted as the major modifiable factor in preventing glaucoma-related damage. Excessive pressure mechanically strains the optic nerve head, compromising axonal flow and triggering cell death. Progressive nerve fiber loss manifests gradually, with “tunnel vision” marking advanced disease. Treatment aims to reduce IOP into a safer range, slowing or halting optic nerve deterioration and preserving visual function.
Traditional Management Approaches
Most initial therapies rely on topical medications, such as prostaglandin analogs, beta-blockers, alpha agonists, or carbonic anhydrase inhibitors. While often highly effective when used correctly, daily drops pose adherence challenges. Many patients, particularly older adults with comorbidities, inadvertently skip doses, diminishing long-term efficacy. Laser therapies (like selective laser trabeculoplasty) and surgical interventions (e.g., trabeculectomy, shunts, minimally invasive glaucoma surgeries) are additional options, though each carries unique risks, recovery times, and viability windows.
Why a Sustained-Release Implant?
Durysta harnesses bimatoprost, a potent prostaglandin analog, in an implant form that slowly releases medication. This addresses some key shortcomings of daily drops:
- Compliance: A single procedure ensures medication delivery for months, drastically reducing or even negating the need for daily self-administration.
- Steady State Delivery: Instead of periodic spikes in medication concentration after each drop, the implant offers a continuous release, likely smoothing out IOP fluctuations.
- Reduced Preservative Exposure: Chronic topical drop use can irritate the ocular surface due to preservatives like benzalkonium chloride (BAK). Durysta’s in-eye mechanism bypasses such repeated exposures.
- Potentially Better Outcomes: Consistent IOP control is strongly tied to stable or improved visual function, less nerve fiber damage, and fewer disease-related complications.
Bimatoprost’s Established Track Record
Bimatoprost is among the most potent glaucoma drugs, acting primarily via enhancing uveoscleral outflow (and to some extent, trabecular outflow). Through morphological changes in the ciliary muscle and extracellular matrix remodeling, it streamlines fluid drainage from the eye, exerting strong pressure-lowering benefits that typically surpass many older therapies.
The transition from a drop to an implant approach constitutes an evolution rather than an unproven novelty. Clinicians and patients alike benefit from bimatoprost’s robust data on efficacy and safety, augmented by the new convenience afforded by sustained delivery. However, as with any emerging therapy, prudent clinical judgment and patient-specific assessment remain vital. Certain individuals, especially those with compromised corneal endothelium or severe advanced disease, need careful evaluation before proceeding.
Durysta’s Role in a Busy Treatment Landscape
Glaucoma therapy is not one-size-fits-all. The Durysta implant might serve as a standalone solution for patients with mild to moderate glaucoma who desire a reduced medication burden, or it may combine with other therapies in more advanced or refractory cases. In many scenarios, substituting daily prostaglandin drops with a bimatoprost implant significantly decreases medication complexity. For those living with physical or cognitive impairments, the elimination of daily drop routines can mark a dramatic improvement in disease management consistency.
In short, Durysta emerges as both a clinically potent and logistically appealing option. Yet broad acceptance also requires deeper comprehension of the therapy’s design, method of use, benefits, and potential risks. The sections below explore the implant’s practicalities, from underlying mechanisms and insertion techniques to follow-up protocols and real-world results.
2. How Durysta Revolutionizes Bimatoprost Delivery
Foundations of Prostaglandin Analog Therapy
Bimatoprost belongs to the prostaglandin analog class, known for powerful IOP-lowering properties. Prostaglandin analogs increase aqueous humor outflow through remodeling ocular tissues. Their daily-drop convenience and favorable side-effect profile made them a first-line choice for many glaucoma patients long before sustained-release versions emerged.
However, the success of daily drops hinges on patient diligence. Missed or irregular doses erode therapy benefits, leading to unsteady pressure control. Over time, fluctuations in IOP can accelerate optic nerve injury, something that long-term therapy aims to avoid. If these difficulties are severe, some individuals turn to more invasive measures like laser or incisional surgery.
Implant Composition and Biodegradability
Durysta harnesses biodegradable polymers—commonly derived from compounds like poly(lactic-co-glycolic) acid (PLGA)—that house the bimatoprost molecule in a tiny pellet. After injection into the anterior chamber, the pellet slowly dissolves, ensuring a consistent drug release. By the time it is largely dissolved (usually by 4–6 months), the IOP-lowering effect has typically peaked and gradually waned. The body harmlessly breaks down the residual polymer fragments, so no implant removal procedure is needed.
Medication Release Dynamics
Prostaglandin analogs modulate various ocular structures, but the ciliary muscle’s extracellular matrix is the primary target. Durysta’s design is meant to keep bimatoprost concentrations stable, minimizing daily troughs or spikes. By sustaining a moderate yet effective dose in the eye, the implant fosters an environment conducive to steady fluid outflow.
Some patients experience near-immediate benefits, with measurable IOP drops within days to weeks. Peak effect typically stabilizes within the first month, continuing well into the next several months. The removal of daily user input largely eliminates adherence-related dips in pressure control. Notably, real-world response can vary based on factors like severity of the disease, individual ocular anatomy, and prior medication history.
Corneal Endothelium Considerations
Any device placed in the anterior chamber raises questions about corneal health. The corneal endothelium is a monolayer critical for regulating corneal hydration. Repeated or extended contact from foreign materials poses theoretical risks of endothelial cell loss or decompensation. Extensive trials indicate that Durysta is generally well-tolerated, but repeated implants or eyes with compromised endothelial counts require scrutiny. In the majority of documented cases, single or limited Durysta administrations do not produce clinically significant endothelial harm, although continued monitoring remains prudent.
Comparisons with Topical Bimatoprost
While the active ingredient is the same, Durysta differs in several ways:
- Elimination of Preservatives: Chronic exposure to preservatives in daily drops can damage the ocular surface. The implant spares patients from that repeated irritant.
- Potentially Fewer Systemic Effects: Eye drops sometimes drain through the nasolacrimal duct, entering systemic circulation. An implant delivering medication locally in the eye may decrease such absorption.
- No Patient Handling: Daily drop instillation demands dexterity, proper timing, and consistent technique. An implant bypasses these pitfalls once placed.
For a subset of patients who responded well to topical bimatoprost but found daily use taxing, Durysta offers a near-equivalent therapy in sustained-release form. Others previously intolerant to bimatoprost’s side effects might still experience them to a degree. Key symptoms of prostaglandin analogs—like hyperemia or eyelid/iris pigmentation—could still manifest with an implant, albeit potentially less pronounced if local concentrations differ from topical usage patterns.
Mechanisms of Longevity
Given that bimatoprost fosters structural remodeling, the benefits to outflow may persist somewhat beyond the polymer’s dissolution. Some patients maintain lowered pressures for a time even after the drug reservoir is spent. However, the official claim of six-month control is based on average, conservative estimates. Not every patient achieves the upper limit of that timeframe, and many watch for signs of rising pressure around the four- to five-month mark. When IOP begins creeping back up, either another Durysta injection or alternative therapy adjustments may be needed.
In this sense, Durysta acts less like a permanent fix and more like a durable method of delivering an established therapy. The convenience factor cannot be overstated—demanding little to no maintenance from the patient, beyond standard follow-up appointments and typical ocular hygiene.
With the fundamentals clear, focusing on how Durysta is deployed in clinical practice further illuminates both its merits and the reasons for caution. As with any device-based therapy, the procedure requires technical skill, and thorough pre- and post-operative assessments are key to success.
3. Practical Use: From Implantation to Daily Living
Indications and Preoperative Assessments
Before recommending Durysta, ophthalmologists conduct a comprehensive eye exam. Gonioscopy confirms an adequately sized and open anterior chamber angle to accommodate the device. Corneal pachymetry or specular microscopy may assess endothelial cell counts, ensuring no significant compromise that might predispose to corneal complications. Additionally, a thorough glaucoma workup—optic nerve imaging, visual field tests, and baseline IOP readings—guides expectations for target pressure.
Durysta is suitable for:
- Open-Angle Glaucoma: The majority of patients who struggle with daily drops, or whose IOP remains borderline on existing therapies.
- Ocular Hypertension: Elevated IOP without diagnosed nerve damage, but still needing consistent management.
- Comorbid Conditions: Cases where frequent topical medication is contraindicated or poorly tolerated.
Yet, patients with advanced disease who need very low target IOP, or with significant endothelial pathologies, might be better served by alternative or complementary treatments. As always, balancing benefit and risk remains central to therapy choice.
Implantation Technique
The Durysta insertion typically occurs in an outpatient setting, often right in a clinical office:
- Numbing and Sterile Prep: Topical anesthetic ensures comfort. The eye is cleaned with antiseptics to reduce infection risk.
- Applicator Positioning: The physician uses a specialized single-use applicator containing the implant. A tiny corneal incision (often sub-1 mm) is created or the injector is gently placed at the corneal or limbal interface.
- Injection: With careful control, the implant is deposited into the anterior chamber’s inferior angle or similar space where it won’t contact the cornea.
- Immediate Check: The surgeon inspects the anterior chamber under slit-lamp or gonioscopic view, verifying correct positioning and lack of complications.
Patients generally report minimal discomfort, and the procedure usually completes in a few minutes. Post-procedure, some individuals might sense a mild foreign-body feeling, but that often subsides quickly. An eye shield or gentle instructions (like avoiding eye rubbing) might be advised for a short while.
Postoperative Medications and Follow-Up
Depending on physician preference and any coexisting ocular issues, a short course of topical steroids or NSAIDs may be prescribed to manage mild inflammation. The first follow-up visit often falls within one to two weeks to check:
- Implant Position: Confirming no migration near the cornea.
- IOP Trends: Some patients experience an early spike or dip, though bimatoprost generally lowers IOP.
- Corneal Clarity: Ruling out microcystic edema or other disruptions from the procedure.
Subsequent appointments might occur monthly or as clinically warranted. As weeks pass, patients or caregivers monitor for changes in vision, eye redness, or discomfort. In many instances, a successful implant means a substantial cut in daily drop usage, possibly discontinuing other prostaglandin analogs or even multiple drug classes if the pressure remains adequately controlled. However, certain individuals still require adjunctive therapy for optimal outcomes.
Lifestyle and Activity Modifications
Unlike some more invasive surgeries, Durysta implantation imposes minimal daily life restrictions. Most patients resume normal routines swiftly:
- Work and Exercise: Normal tasks can typically continue, although high-intensity activities (like contact sports) may be avoided briefly to let the incision fully heal.
- Travel: Not having to worry about traveling with multiple eye-drop bottles can be a relief for those who live mobile lifestyles or frequently go on extended trips.
- Concurrent Eye Conditions: If the patient has cataract or macular pathology, the presence of a Durysta implant rarely complicates typical treatments for these conditions. Nonetheless, practitioners coordinate care to ensure procedures like cataract surgery proceed safely without interfering with the implant.
Managing Residual Drops
Some individuals may be on a multi-drug regimen for moderate or advanced glaucoma. After Durysta, the clinician might systematically taper the patient off certain drops or reduce their frequency, observing IOP response. If stable control persists without pressure spikes, many can lighten or eliminate daily drop burdens, greatly enhancing quality of life.
For others, the addition of Durysta may partially relieve the medication load, but not entirely. Even partial relief from one or two daily drops can help, especially if dryness or ocular surface disease is an issue. Ultimately, custom-tailored regimens ensure each patient’s target IOP is met without unnecessary therapy.
Longevity and Reimplantation
Although the official labeling touts up to six months of IOP control, real-world data indicate variability. Some see robust control beyond that timeframe, while others need earlier re-treatment. Currently, repeat implants remain somewhat constrained by regulatory guidelines and caution regarding corneal health over multiple injections. However, many specialists anticipate these restrictions could evolve as more evidence supports repeated usage.
The question of indefinite repetitive Durysta implants is still under study. Preliminary experiences with multiple implants in the same eye show promise for sustaining long-term control, but prospective trials exploring long-term endothelial effects, cumulative polymer presence, and potential device migration or rotation are ongoing. This underscores the dynamic nature of the therapy, with more refined protocols likely to emerge.
4. Latest Research Insights: Confirmed Efficacy from Clinical Studies
Key Clinical Trials
Numerous studies back Durysta’s potency and safety. Two pivotal randomized controlled trials known under the ARTEMIS umbrella stand out, collectively enrolling hundreds of patients with open-angle glaucoma or ocular hypertension. Participants receiving a single bimatoprost implant demonstrated:
- Durable IOP Reduction: Average decreases of around 5–8 mmHg from baseline, a range deemed clinically significant and comparable to daily topical bimatoprost.
- Extended Duration: Pressure remained favorably lowered for four to six months in a large portion of the subjects, validating the concept of sustained therapy.
- Side Effect Profiles: Mostly mild to moderate, including mild eye irritation and transient hyperemia. Serious adverse events, such as severe corneal edema or infection, were rare.
Comparative Effectiveness vs. Eye Drops
Though direct trials pitting Durysta exclusively against daily bimatoprost are limited, broad data from the ARTEMIS studies and real-world anecdotal evidence show similarly robust IOP control without daily user compliance issues. This positions the implant as a near-equivalent to eye drops from an efficacy standpoint, but with the potential advantage of consistent delivery and reduced day-to-day variability.
Some open-label extension studies tracked patients beyond six months. While the effect typically diminished as the implant fully resorbed, many participants retained partial pressure-lowering benefits up to eight or nine months. This encourages further evaluation of reimplantation schedules to maintain continuous IOP control.
Long-Term Observations
As Durysta transitions from clinical trials to broader usage, long-term safety outcomes are gathering. Minimal increases in corneal endothelial cell loss are reported over typical six-month intervals. Ongoing registries track repeated implant placements in selected patients, exploring cumulative effects on the cornea, angle structures, and ocular tissues. Early signals suggest no major spike in complications, but more extensive data remain essential.
Patient Satisfaction Surveys
Patient-centric measures of success, like convenience and daily-living satisfaction, are especially relevant for chronic conditions like glaucoma. Preliminary surveys indicate that individuals who receive Durysta appreciate the freedom from daily dosing, reduced discomfort from eye-drop preservatives, and fewer disruptions to travel or work. Many also report a sense of emotional relief, feeling more confident that their IOP remains stable without constant self-monitoring.
Additionally, caregivers for older patients note a decrease in the time and effort spent administering or reminding about drops, further boosting acceptance. These intangible benefits underscore the potential for greater adherence, albeit in a form that no longer relies on patient self-administration but rather on in-office procedures.
Special Cases in Clinical Research
Of interest are subpopulations such as:
- Pseudoexfoliative Glaucoma: Some early data hint at positive outcomes in pseudoexfoliation, where the ocular structures can be more reactive. The consistent delivery of medication may offset periods of increased outflow resistance.
- Normal-Tension Glaucoma: Durysta’s role in normal-tension cases remains less established, though any therapy that reduces IOP further can potentially slow disease in borderline pressurized eyes.
- Coexisting Cataract or Comorbidities: Some surgeons explore Durysta placement during or following cataract surgery, effectively combining two procedures to streamline care. Preliminary experiences suggest good synergy, though robust trial data are pending.
Economic Impact Studies
From a public health perspective, glaucoma stands as a major cost driver due to lost productivity, frequent medication refills, and advanced surgeries if the disease progresses. While an implant procedure is pricier initially, many health economists posit that improved adherence and fewer complications might reduce overall costs, especially if patients avoid more invasive procedures down the line. Studies modeling cost-effectiveness typically factor in both direct costs (drug, administration) and indirect ones (missed work, caretaker burden). Preliminary findings often favor innovative sustained-release solutions, though wide-scale adoption depends on insurance coverage complexities and budgetary constraints within healthcare systems.
All told, the research to date consistently reaffirms Durysta’s efficacy and safety for a broad swath of patients with open-angle glaucoma or ocular hypertension. The therapy yields robust, extended IOP control that parallels daily bimatoprost in performance. Yet, real-world acceptance also hinges on clarifying potential side effects and meticulously tracking ocular changes. Additional data from repeated usage, extended follow-ups, and cost analyses will further refine how Durysta fits into the modern glaucoma care arsenal.
5. Evaluating Real-World Effectiveness and Safety
Persistent IOP Reduction: Clinical Corroboration
Real-world outcomes echo the trial data, with many ophthalmologists reporting stable or improved IOP levels over four to six months after a single Durysta insertion. Because this approach eliminates the day-to-day variability of topical compliance, significant fluctuations become less likely. Patients who previously struggled to maintain consistent drop schedules often notice markedly smoother IOP profiles, occasionally lowering their risk of progressive nerve damage.
Likewise, some clinics find that combining Durysta with MIGS or prior selective laser trabeculoplasty (SLT) can produce complementary results. Where single strategies may not achieve the desired pressure threshold, layering advanced therapies can yield synergy. For instance, a patient with borderline IOP control post-laser might see a further drop once the implant is in place, forestalling or postponing more invasive surgeries.
Clinical Safety Signals
While the general safety profile is reassuring, vigilance remains paramount. Reported side effects range from mild hyperemia or itching to occasional issues like:
- Transient Increased IOP: Some eyes experience a short-lived postoperative spike, typically controlled through standard IOP-lowering drops or observation.
- Corneal Endothelial Observations: Over multiple implants, some concerns about endothelial cell stability remain theoretical but under ongoing observation.
- Inflammation and Discomfort: Injection-based procedures can cause short-term inflammation, usually subdued by brief topical steroid use.
- Rare Device Dislocation: The pellet is designed to rest in the inferior angle, but movements can occur if the chamber is shallower than anticipated or if the patient engages in intense physical activities early on.
Specific cases of severe complications—like endophthalmitis or permanent corneal damage—appear extremely rare in the literature, typically tied to preexisting conditions or nonstandard technique. From a systemic perspective, limited absorption of bimatoprost outside the eye means the usual concerns about systemic side effects are minimal.
Corneal Endothelium Monitoring
Ongoing follow-ups frequently include specular microscopy or endothelial cell counts, especially if repeated implants are anticipated. Although single placements rarely show significant cell loss, the impetus for close checks is wise. Notably, eyes with compromised endothelium from prior surgeries (e.g., corneal transplants, advanced Fuchs’ dystrophy) warrant extra caution, as any further stress could exacerbate edema or decompensation.
Extended-Use Considerations
Data on multiple sequential Durysta implants remain limited in scope. Some ophthalmologists, however, have performed second or even third injections after the initial effect wanes, especially in patients who have thrived on sustained bimatoprost release. Preliminary anecdotal experiences are positive, with no dramatic surge in complication rates, but formal long-term studies are in progress.
Patient-Reported Outcomes in Practice
Subjective accounts highlight that many individuals appreciate the freedom from daily drops. Some remain vigilant about noticing subtle changes in their vision or ocular comfort, scheduling timely checkups as recommended. The psychological relief from not needing daily medication cannot be overstated—particularly for older adults or those already managing multiple prescriptions. By reducing “treatment fatigue,” Durysta fosters better disease acceptance and overall contentment with care.
At the same time, certain patients discover that while the implant reduces or eliminates one medication, they still need adjunct drops for complete control. This scenario is especially common among advanced or aggressive glaucomas needing extremely low IOP targets. Even partial medication relief, though, can significantly lessen ocular surface problems and daily regimen complexities.
Overall, real-world feedback generally aligns with trial results, confirming Durysta’s potential to transform how stable or moderately progressive glaucoma is managed. Even so, cost factors and coverage policies play a defining role in practical accessibility, which the subsequent section addresses.
6. Therapy Price: Navigating Costs and Financial Options
Durysta’s innovative design and injection procedure often entail higher upfront expenses than generic eyedrops. Depending on practice setting, a single implant can cost several hundred to over a thousand dollars. Insurance coverage varies, with some plans paying partly or fully if the procedure meets medical-necessity criteria. A few clinics offer payment plans for self-paying individuals. Manufacturer coupons or patient-assistance programs may reduce costs further. For many, the long-term savings in reduced medication refills and fewer specialist visits can offset the initial outlay, particularly when accounting for intangible benefits like better adherence and peace of mind.
Disclaimer:
This article is for educational purposes only and does not replace professional medical advice. Always consult an eye care specialist to determine the best treatment plan for your individual situation.
We encourage you to share this information with friends, family, or others who might benefit. Use our Facebook and X (formerly Twitter) share buttons or any other method you prefer—together, let’s broaden awareness about Durysta and advance the conversation on innovative glaucoma care!