Glaucoma poses a significant challenge for millions of individuals worldwide, as it often requires daily medication regimens to control intraocular pressure and prevent irreversible vision loss. Patients and clinicians have long grappled with the need for consistent adherence to eye drops and the associated risk of forgetting doses or experiencing side effects. With these hurdles in mind, the development of new drug-delivery mechanisms that can sustain therapeutic levels of medication over extended periods represents a major milestone in glaucoma care.
The iDose TR Implant stands out as a game-changing intervention by offering continuous, long-term delivery of glaucoma medication. Designed to remain effective for up to three years, this implantable device reduces the burden of daily drops and helps ensure consistent intraocular pressure control. Below, we explore the unique attributes of the iDose TR, examine its growing body of supporting research, and discuss both its clinical application protocols and cost considerations.
Unraveling the iDose TR Implant’s Key Advantages
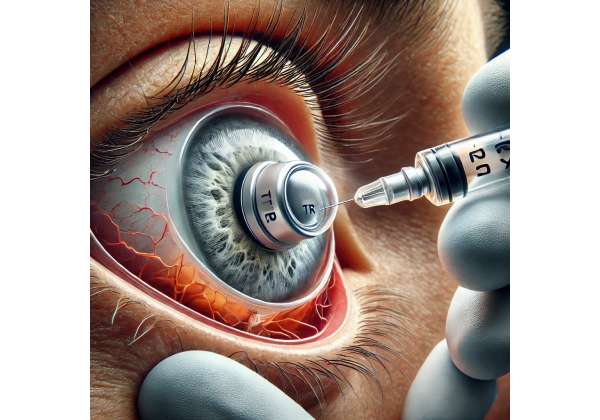
The iDose TR Implant is a tiny, intraocular device developed to release a steady flow of medication directly into the anterior chamber of the eye over an extended timeframe—often cited as three years. Its primary purpose is to maintain therapeutically effective levels of a potent anti-glaucoma drug while eliminating many of the compliance obstacles tied to daily eye-drop regimens. By focusing on direct, sustained drug delivery, this novel approach seeks to improve patient outcomes and overall adherence rates in glaucoma management.
A Fresh Perspective on Medication Delivery
Traditionally, patients diagnosed with open-angle glaucoma or ocular hypertension rely on daily or multiple daily instillations of topical eye drops such as prostaglandin analogs, beta-blockers, or carbonic anhydrase inhibitors. While these drops are relatively effective at reducing intraocular pressure (IOP) in theory, real-world adherence can be inconsistent. Reasons include side effects such as eye irritation, the complexity of multiple daily administrations, or simply forgetting to apply medication on a strict schedule. This phenomenon raises the risk of disease progression.
The iDose TR Implant introduces a paradigm shift. By placing the implant inside the eye, it bypasses potential surface-level irritations and ensures a stable, controlled release of medication. This approach addresses key limitations:
- Continuous Therapeutic Effect: Instead of daily peaks and troughs associated with eye drop usage, iDose TR can maintain more consistent IOP control.
- Reduced User Error: Once implanted, the risk of forgetting a dose or applying medication incorrectly is significantly diminished.
- Lower Frequency of Side Effects: Since it can use a smaller amount of drug delivered directly to the target site over time, some patients may experience fewer systemic or surface-level adverse effects.
Anatomical Fit and Ease of Placement
The iDose TR Implant is designed to be small enough to fit seamlessly within the anterior chamber, anchoring in the trabecular meshwork. This anchoring system ensures the device remains stable over its intended lifespan. Its robust structure and biocompatible materials provide both durability and safety, preventing leakage or dislocation. Moreover, ophthalmic surgeons often report that the procedure for placing iDose TR is minimally invasive, comparable in complexity to typical MIGS (minimally invasive glaucoma surgeries).
Because the implant is positioned where aqueous humor naturally drains, it can effectively deliver a drug such as travoprost—an agent known for its proven efficacy in lowering IOP—directly to the tissues that regulate fluid outflow. By supplying the medication exactly where it is needed, the device capitalizes on localized therapy while minimizing exposure to the rest of the eye’s delicate structures.
Built-In Medication Reservoir
Central to iDose TR’s functionality is its reservoir chamber, which holds the active compound. The implant’s membrane technology controls the rate at which the medication diffuses into the eye’s interior. Over time, the reservoir depletes slowly. Once the drug load is fully expended—commonly after several years—an ophthalmologist can remove and replace the implant if ongoing therapy is needed. This capacity for eventual removal and reimplantation is vital for patients with chronic conditions like glaucoma, which demand lifelong management.
Addressing Patient Adherence and Quality of Life
Adherence remains an ever-present concern in chronic eye diseases, as missed doses and inconsistent use can sharply undermine treatment outcomes. By automating the drug-delivery process, the iDose TR Implant reduces the need for daily vigilance and can preserve or even improve vision quality. Furthermore, patients no longer need to juggle multiple eye drops for a single condition, alleviating both the logistical and psychological burden.
This boost in adherence can translate into stable or improved visual fields, a crucial metric in measuring glaucoma progression. Stable visual fields mean that nerve damage is either halted or slowed, helping patients maintain better functional vision in the long term. Consequently, many experts view the iDose TR as a major step forward, particularly for populations at high risk of disease worsening due to erratic medication use.
Anticipated Longevity
While the nominal timeframe is around three years, actual longevity can vary based on individual ocular parameters, such as aqueous humor turnover rate and the specific dosage needed to keep IOP within a safe range. However, multi-year coverage from a single implant stands out as a tremendous advantage over conventional daily treatments. Patients can enjoy extended periods of stable IOP control without the hassle of frequent medical visits or prescription refills. When the device nears depletion, surgical exchange offers a renewed reservoir of medication, sustaining therapy indefinitely.
Given these features, it is clear how the iDose TR Implant sets itself apart from prior glaucoma solutions. By focusing on both convenience and robust IOP control, it exemplifies a patient-centric design that addresses many of the real-world hurdles plaguing long-term ocular drug administration. Beyond that, it paves the way for future implants and technologies that leverage sustained-release systems to transform how we treat chronic eye conditions.
How iDose TR Is Administered and Key Treatment Steps
While the concept behind the iDose TR Implant is innovative, its surgical procedure and follow-up care remain relatively straightforward, especially in the context of current minimally invasive glaucoma surgery (MIGS) techniques. Understanding the key steps in placement and the postoperative protocols is vital for any ophthalmologist considering offering this therapy—and for patients interested in learning what to expect.
Candidacy and Preoperative Evaluation
Determining whether a patient can benefit from iDose TR starts with a comprehensive eye exam. Patients with open-angle glaucoma or ocular hypertension who struggle with compliance—whether due to forgetfulness or adverse events from eye drops—stand to benefit most. These individuals may also have mild-to-moderate disease progression and need consistent IOP control to prevent further optic nerve damage.
During the evaluation, ophthalmologists look for any anatomical variations in the trabecular meshwork or anterior chamber that could complicate implant insertion. Gonioscopic examinations, optic nerve imaging (like OCT), and visual field tests help confirm the diagnosis and gauge how advanced the patient’s glaucoma is. Any concurrent conditions, such as corneal pathology or uveitis, may influence the decision to proceed or require additional planning.
Surgical Procedure and Implant Placement
iDose TR’s insertion is typically done in an outpatient setting, often under local anesthesia. The surgical steps generally include:
- Minimal Incision: A small corneal or scleral incision is created to allow instrument access to the trabecular meshwork region.
- Visualizing the Drainage Angle: Surgeons use a gonio lens or microscope to clearly view the angle. This ensures precise, stable placement of the implant.
- Implant Insertion: The iDose TR device is carefully guided to its anchoring point in the trabecular meshwork. Its design helps ensure it remains secure once positioned.
- Incision Closure: Depending on technique and incision size, the wound may self-seal or require minimal suturing. The small incision nature helps speed up postoperative recovery.
Since the process is minimally invasive, patients often experience less postoperative discomfort than with traditional filtering surgeries. Many can resume daily activities after a short period of rest. However, each case is unique; some individuals may require closer monitoring, particularly if they have complex ocular histories.
Immediate Postoperative Care
Once the implant is in place, standard postoperative measures help mitigate inflammation and reduce infection risk. Patients typically use a short course of antibiotic drops along with mild topical steroids or non-steroidal anti-inflammatory drugs (NSAIDs) as directed by their surgeon. The eye must be protected from accidental trauma, so clinicians may recommend wearing a shield or avoiding strenuous activities for the first few days.
Follow-up visits in the first week or two are critical to assess how effectively the implant is lowering IOP. Surgeons also verify that the device remains in position and that there are no signs of complications like significant inflammation or infection. Any postoperative spikes in intraocular pressure, while uncommon, are manageable with short-term medication or adjustments to existing therapy. In many instances, the gradual release of travoprost from the implant stabilizes ocular pressure effectively, often allowing a reduction or discontinuation of traditional eye drops.
Long-Term Management and Monitoring
A unique aspect of iDose TR is its longevity. Once the patient settles into the steady drug-release phase, fewer routine doctor visits may be necessary compared to traditional regimens. Nonetheless, periodic check-ups remain essential to evaluate:
- Intraocular Pressure: Ensuring the IOP remains within target range, preventing optic nerve damage.
- Implant Integrity: Confirming the device has not dislodged or degraded.
- Signs of Disease Progression: Visual fields and OCT imaging can detect early changes in optic nerve health.
Given that the implant lasts around three years, patients can expect a discussion about replacement or alternative options once the medication reservoir is close to depletion. Extraction and reimplantation typically follow a process similar to the initial surgery, maintaining continuity of care. Some surgeons may opt for concurrent procedures if cataract surgery or other MIGS interventions are indicated at a later point, capitalizing on a single operative event to address multiple ocular needs.
Combining iDose TR with Other Therapies
Although the iDose TR Implant provides a significant leap in sustained medication delivery, certain patients may still require supplemental eye drops or laser treatments, especially if they have advanced glaucoma that necessitates additional interventions. In such cases, the synergy between iDose TR and ancillary measures can yield optimal pressure control. The continuous, stable release of medication from the implant might even enhance the efficacy of adjuvant therapies by providing a consistent baseline IOP reduction.
Moreover, combining iDose TR with MIGS procedures like the Hydrus Microstent or trabecular meshwork stents could be beneficial for a subset of patients. However, such combined strategies are still being explored clinically, and outcomes can vary depending on patient anatomy and disease severity.
Potential Complications and Their Management
While the iDose TR procedure boasts a relatively safe profile, complications can arise. These may include:
- Transient IOP Spikes: Occasional postoperative pressure elevations can occur due to inflammation or angle-based changes. Prompt management with topical or oral anti-hypertensives usually resolves these.
- Iritis or Uveitis: Mild inflammation in the anterior segment, treatable with topical corticosteroids.
- Device Malposition: Rarely, the implant may shift or fail to anchor properly, necessitating repositioning.
- Infection: As with any intraocular procedure, strict aseptic technique and antibiotic prophylaxis reduce the risk.
Such issues are typically manageable with close monitoring. The majority of patients experience minimal discomfort, and many find the insertion process far less intimidating than conventional surgeries like trabeculectomy or tube shunt implantation.
All told, the iDose TR’s insertion, once reserved for research settings, is swiftly entering mainstream practice as practitioners adopt this novel method. By following established protocols, surgeons can unlock the many benefits of a three-year medication reservoir in a single, minimally invasive operation, granting individuals with glaucoma greater independence from daily drops and reinforcing consistent IOP control.
Notable Findings and Emerging Research on iDose TR
As the iDose TR Implant transitions from clinical trials to real-world settings, a wealth of research underscores its potential to shape the future of glaucoma management. Several long-term studies, sponsor-led trials, and independent investigations offer insights into how effectively the device controls intraocular pressure while preserving patient quality of life.
Focal Clinical Trials and Their Outcomes
One key milestone in the iDose TR story is a series of pivotal clinical trials demonstrating both the safety and sustained efficacy of the implant. In these trials, patients who received iDose TR often achieved significant IOP reductions comparable to or exceeding daily prostaglandin eye drops. Importantly, these gains persisted for extended follow-up periods—two, three, or more years—without the need for frequent re-treatment.
The data consistently show a marked decline in peak IOP measurements, a critical factor in staving off optic nerve damage. Investigators also highlight reduced fluctuations in diurnal IOP, reflecting a more continuous drug presence within the eye. This stability can be crucial, given that rapid or pronounced swings in eye pressure have been linked to faster disease progression.
Comparing iDose TR to Traditional Eye Drops
Beyond the raw numbers of IOP decrease, researchers point to dramatic improvements in patient adherence and overall satisfaction. When a standardized questionnaire was used to gauge patient convenience, those with the iDose TR frequently expressed relief at not having to manage multiple eye drops daily. Some also reported fewer ocular surface complaints—such as irritation or dryness—possibly because fewer topical medications were necessary.
In direct head-to-head comparisons, iDose TR outcomes in lowering IOP often matched that of established topical therapies like travoprost or latanoprost. Yet, the sustained aspect stands out as a competitive advantage. Maintaining near-constant drug levels can enhance nerve protection, reduce the risk of missed doses, and may potentially mitigate the chances of disease progression. While certain subpopulations may still require additional therapy, the overall trial results paint a favorable picture for broad adoption.
Safety and Tolerability Observations
Clinical trials also closely monitored adverse events to create a transparent risk profile. Incidences of postoperative complications—like significant hyphema or corneal edema—remained low and generally comparable to other MIGS procedures. Similarly, rates of device dislocation or mechanical failure were notably rare, though long-term vigilance is encouraged.
Regarding systemic safety, the localized nature of iDose TR helps keep the drug primarily within the eye. Investigators did not note increased systemic side effects tied to the medication, providing reassurance for patients worried about cardiovascular or respiratory impacts sometimes associated with certain topical glaucoma drops.
Extension Trials and Real-World Studies
A hallmark of cutting-edge medical products is the extension study—trials that extend beyond initial timeframes to see how well benefits hold up. For iDose TR, these extension phases reveal that some implants maintain substantial efficacy even past the nominal three-year point, depending on the specific drug formulation and patient physiology. However, each patient’s experience can differ, and discussions about replacement or alternative therapy remain critical once the implant’s reservoir nears depletion.
Meanwhile, real-world data registries are taking shape. Clinicians around the globe are logging patient outcomes, capturing not only IOP measures but also quality-of-life indicators like ease of daily activity and freedom from frequent medication reminders. This real-world evidence can sometimes reveal trends not clearly evident in controlled trial environments—such as how the implant performs in diverse populations or among older adults managing multiple systemic conditions.
Prospective Innovations
As iDose TR gains traction, research groups and the manufacturer have started evaluating potential improvements, including:
- Alternative Drug Formulations: While travoprost is currently standard, other agents—like combination therapies or newer molecules—could be explored for extended release in a similar implant.
- Adjustable or Refillable Designs: Some scientists envision implants that can be refilled in-office, potentially prolonging the device lifespan indefinitely and sparing patients a second surgical procedure.
- Smart Monitoring: Future concepts include sensors that track reservoir levels or real-time IOP, relaying data to physicians via digital apps. This synergy could further refine individualized care.
Additionally, parallel research on microshunt technologies, gene therapy, and other extended-release systems highlights a renaissance in glaucoma innovation. While each approach aims to protect the optic nerve, the iDose TR stands out for its proven track record, relative ease of placement, and robust reduction in daily medication requirements.
Overall, the surge of positive trial data and ongoing investigations confirms that iDose TR is more than a fleeting novelty. It heralds a transition to a future where controlling IOP does not hinge upon patient vigilance with daily eyedrops. Instead, implants and sustained-release systems may ultimately set a new standard of care—streamlined, continuous, and deeply focused on preserving vision for the long haul.
Evaluating Efficacy and Safety
For anyone considering the iDose TR Implant—clinicians, patients, or caregivers—a firm grasp of its performance metrics and potential risks is essential. While the therapy’s design and emerging research appear highly promising, contextualizing efficacy and safety alongside standard glaucoma treatments fosters more informed, individualized decision-making.
Established Benefits in IOP Control
Multiple peer-reviewed studies agree that the iDose TR offers substantial reductions in intraocular pressure, often mirroring or surpassing the effects of daily topical travoprost. Not only is the magnitude of IOP lowering on par with established therapies, but the delivery method also provides greater uniformity in medication concentration. The result is:
- Stable Baseline Pressure: Minimizing daily peaks and troughs can reduce the likelihood of harmful fluctuations.
- Fewer Progression Worries: Individuals with moderate risk or early optic nerve damage may benefit most from the consistency iDose TR delivers.
- Sustained Results: Clinical trials spanning 24 to 36 months consistently report beneficial IOP levels within target ranges. Some observational data even indicate effective IOP control beyond three years, though further research is needed to confirm these longer-term results in broad populations.
Enhanced Patient Lifestyle and Compliance
In the realm of chronic disease management, adherence can be as pivotal as drug efficacy. The iDose TR’s ability to diminish reliance on daily drops significantly boosts the likelihood of steady disease management. Among older adults, individuals with dexterity issues, or those with cognitively demanding routines, relief from remembering or administering eye drops may help reduce stress and improve day-to-day functioning. This sense of empowerment can indirectly bolster overall well-being, as patients feel more in control and less burdened by their condition.
Potential Risks and Complications
No procedure is without possible downsides. For the iDose TR, recognized complications typically fall under categories familiar to most eye surgeries:
- Procedural Complications: Rare but possible events during insertion include bleeding (hyphema), corneal abrasions, or mild trauma to adjacent eye structures.
- Device Stability: While anchors are designed to secure the implant in place, dislocation or partial migration can occur in very rare instances. Surgeons can reposition the implant if discovered early.
- Postoperative Inflammation: Mild to moderate anterior segment inflammation (iritis) may manifest during the first week or two. This is usually well-managed with topical steroids and resolves quickly.
- Infection: As with any intraocular procedure, strict sterile conditions and follow-up care keep infection rates extremely low, but it cannot be completely discounted.
Yet when placed in the context of other minimally invasive glaucoma surgeries—and especially in comparison to more invasive filtering procedures or tube shunts—the iDose TR retains a favorable safety profile. The short surgical time, minimal incisions, and proven drug design reflect a strategy that prioritizes patient comfort and long-term ocular health.
Considerations for Specific Populations
While iDose TR is generally indicated for open-angle glaucoma or ocular hypertension, certain groups warrant special attention:
- High-Risk Advanced Glaucoma: Patients with advanced nerve damage or severely elevated IOP might still require combined therapies or additional interventions.
- Children and Adolescents: Pediatric use remains understudied. Younger patients may have different rates of aqueous humor turnover, or they might outgrow the device more rapidly.
- Individuals with Autoimmune Conditions: Those prone to uveitis or inflammatory flare-ups may need a careful approach, ensuring that the presence of an implant does not exacerbate underlying inflammation.
Continued research and growing clinical experience should expand knowledge about how iDose TR interacts with these subpopulations. For now, thorough evaluation and close collaboration between patient and provider remain crucial. Understanding personal risk factors, ocular anatomy, and disease severity typically guides whether iDose TR is the right choice.
Long-Term Safety Outlook
The biggest question for many observers is whether multiple cycles of implant insertion, removal, and replacement could pose cumulative risks. While multi-year data remain encouraging, more extended observation is necessary to fully capture the long-term trajectory. Early adopters who are now approaching the time for a second implant have generally reported smooth re-implantation experiences, with no dramatic increase in complications or diminished efficacy. However, each patient’s journey is individual, and large registries or 5- to 10-year follow-ups would be invaluable in solidifying the therapy’s longevity profile.
Based on current evidence, the iDose TR stands as both a highly effective and consistently safe option for well-selected glaucoma patients. Its strong performance in reducing and stabilizing IOP, along with the convenience afforded by long-lasting medication reservoirs, sets a new standard for how we might manage this chronic, sight-threatening disease. Balancing these benefits against known risks affirms that iDose TR merits serious consideration in the evolving landscape of ocular therapeutics.
Pricing Details and Patient Access
Depending on geographical location, clinic type, and insurance coverage, the cost of iDose TR can vary widely. Some centers charge around \$3,000 to \$6,000 per implant, which typically includes the device itself and standard surgical fees. In locations with broader insurance acceptance, a significant portion of this expense may be covered, especially if the therapy is deemed medically necessary. Out-of-pocket costs can be higher for those paying privately, although many clinics offer financing plans, which help distribute payments over time. Checking with insurance carriers and verifying coverage is vital to avoid unexpected expenses.
Disclaimer: This article is intended for informational purposes only and should not be construed as medical advice. Always consult a qualified healthcare professional for personalized recommendations and treatment decisions.
We invite you to share this article with friends, family, and on social media platforms like Facebook and X (formerly Twitter). Your support helps spread valuable information about the iDose TR Implant, enabling more people to discover this innovative therapy and explore whether it may be a viable option for their glaucoma care. By sharing, you can play a part in advancing accessible, long-term solutions for those seeking better vision health.