What is to Bietti’s Crystalline Dystrophy?
Bietti’s crystalline dystrophy (BCD) is a rare inherited eye disorder that causes crystalline deposits in the retina as well as progressive atrophy of the retinal pigment epithelium (RPE) and choroid. BCD was first described by Italian ophthalmologist Dr. Bietti in 1937 and primarily affects young adults, resulting in progressive vision loss over time. The condition is caused by mutations in the CYP4V2 gene, which is essential for lipid metabolism. BCD is more common in people of East Asian descent, but it can occur in other populations as well. Understanding the nature of BCD is critical for early detection and effective treatment in order to maintain vision and quality of life.
In-Depth Look at Bietti’s Crystalline Dystrophy
Bietti’s crystalline dystrophy is an autosomal recessive genetic disorder, which means that an individual receives two copies of the mutated gene, one from each parent. The CYP4V2 gene mutation disrupts the normal metabolism of fatty acids in the eye, resulting in the formation of crystalline deposits. These deposits, which are primarily composed of cholesterol and other lipids, can be found in the corneal limbus, retina, and, in some cases, the corneal stroma.
Pathophysiology
The pathophysiology of BCD is characterized by complex biochemical processes that result in the accumulation of crystalline deposits and retinal degeneration. The CYP4V2 gene produces a cytochrome P450 enzyme that aids in the metabolism of fatty acids and other lipids. Mutations in this gene disrupt the enzyme’s function, resulting in an abnormal accumulation of lipid metabolites in retinal cells. Over time, these deposits damage and kill cells, particularly in the retinal pigment epithelium (RPE) and choroid.
The RPE is a layer of cells that helps to support the photoreceptors (rods and cones) that are responsible for vision. The RPE is responsible for photoreceptor outer segment phagocytosis, visual pigment recycling, and blood-retina barrier maintenance. In BCD, the buildup of crystalline deposits disrupts these functions, resulting in photoreceptor degeneration and progressive vision loss.
Clinical Manifestations
The clinical presentation of BCD can differ significantly between individuals. However, several key characteristics are frequently observed:
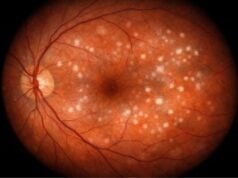
- Crystalline Deposits: BCD is distinguished by the presence of sparkling, yellowish crystalline deposits in the retina. These deposits are frequently visible during a fundoscopic examination and are usually found in the retina’s posterior pole. Similar deposits can also be found in the corneal limbus and, less frequently, in the corneal stroma.
- Retinal Atrophy: The progressive atrophy of the RPE and choroid is a distinguishing feature of BCD. This atrophy causes retinal thinning and photoreceptor loss, resulting in lower visual acuity and peripheral vision.
- Visual Field Defects: As the disease progresses, patients frequently develop visual field defects like scotomas (blind spots) and concentric constriction (tunnel vision). These flaws can have a significant impact on everyday activities and quality of life.
- Night Blindness: Many people with BCD develop nyctalopia, or night blindness, as a result of the loss of rod photoreceptors, which are responsible for vision in low-light conditions.
- Color Vision Deficiency: Color vision may be impaired in the late stages of BCD as cone photoreceptors, which are responsible for color vision, are damaged.
Genetics and Inheritance
BCD is caused by mutations in the CYP4V2 gene, which is located on chromosome 4q35. The CYP4V2 gene encodes an enzyme from the cytochrome P450 family that is involved in the metabolism of many molecules, including fatty acids. The exact function of CYP4V2 in the retina is unknown, but it is thought to play a role in lipid metabolism and homeostasis.
BCD has an autosomal recessive inheritance pattern, which means that the disease requires two copies of the mutated gene to manifest. Carriers, who have only one copy of the mutated gene, do not usually show symptoms, but they can pass the mutation on to their children. Genetic counseling is recommended for BCD-affected families to help them understand the risks and implications of the mutation.
Epidemiology
BCD is a rare condition that has a higher prevalence in East Asian populations, particularly in China and Japan. However, cases have been documented in people of various ethnicities around the world. Although the exact prevalence of BCD is unknown, it is classified as a rare disorder.
Differential Diagnosis
Several other retinal dystrophies can present with similar clinical features to BCD, so differential diagnosis is critical. These conditions include the following:
- Stargardt Disease: The most common form of inherited juvenile macular degeneration, it is distinguished by the accumulation of lipofuscin in the RPE. Unlike BCD, Stargardt disease does not usually have crystalline deposits.
- Retinitis Pigmentosa (RP): RP is a group of inherited retinal dystrophies characterized by progressive peripheral vision loss and night blindness. While RP can cause retinal atrophy, it is not usually associated with crystalline deposits.
- Cystinosis: This metabolic disorder causes the accumulation of cystine crystals in a variety of tissues, including the cornea and retina. Cystinosis, on the other hand, is associated with systemic symptoms such as kidney dysfunction, which are not present in BCD.
- Sjögren-Larsson Syndrome is an inherited condition characterized by ichthyosis, intellectual disability, and spasticity. This syndrome is distinguished from BCD by the presence of systemic symptoms in addition to retinal crystalline deposits.
Progress and Prognosis
The progression of BCD is typically slow but relentless, resulting in gradual vision loss over several decades. Individuals’ severity and rate of progression can vary, with some experiencing significant visual impairment by early adulthood and others retaining useful vision well into old age. The presence of crystalline deposits and the degree of retinal atrophy are important factors in determining prognosis.
BCD primarily affects vision, but it rarely causes complete blindness. Peripheral vision is frequently preserved until late in the disease’s progression, allowing affected individuals to maintain some level of independence. However, the central vision loss and visual field defects associated with BCD can have a significant impact on daily activities such as reading, driving, and facial recognition.
Preventive Tips
- Regular Eye Examinations: Schedule regular eye exams with an ophthalmologist to detect early signs of BCD and track disease progression. Early detection can help to manage symptoms and preserve vision.
- Protective Eyewear: Wear sunglasses with UV protection to protect your eyes from harmful ultraviolet rays, which can worsen retinal damage.
- Healthy Diet: Eat a well-balanced diet high in antioxidants, vitamins, and minerals to promote overall eye health. Fish and leafy green vegetables are good sources of omega-3 fatty acids.
- Don’t Smoke: Smoking can hasten the progression of retinal diseases. Avoiding tobacco smoke is critical for maintaining vision.
- Manage Systemic Health: Keep systemic conditions like hypertension and diabetes under control, as they can have a negative impact on retina health.
- Stress Management: Use stress-reduction techniques like yoga, meditation, and deep breathing exercises to manage stress, which can have an impact on overall health.
- Family History Awareness: If BCD runs in your family, learn about it and seek genetic counseling. Understanding your genetic risk can help with early detection and management.
- Use of Visual Aids: Magnifying glasses, large-print books, and other visual aids can help people cope with vision loss and maintain their independence.
- Stay Informed: Learn more about BCD and its progression. Staying informed can help you make better choices about your eye health and treatment options.
- Regular Physical Activity: Regular physical activity promotes overall health and well-being, which can have an indirect benefit on eye health.
Diagnostic methods
Bietti’s crystalline dystrophy (BCD) is diagnosed using a combination of clinical evaluation, advanced imaging techniques, and genetic testing to confirm the condition’s presence and severity.
Clinical Evaluation
The first step in diagnosing BCD is a thorough eye examination by an ophthalmologist. This includes gathering a thorough patient history to identify symptoms such as night blindness, visual field defects, and a family history of similar conditions. Visual acuity tests are used to determine the level of vision impairment.
Fundus Examination
A fundus examination with an ophthalmoscope allows the ophthalmologist to see the retina. In BCD, characteristic crystalline deposits can be found in the retina’s posterior pole. The presence of these deposits, combined with signs of retinal atrophy and choroidal sclerosis, aids in the diagnosis.
Optical Coherence Tomography(OCT)
Optical Coherence Tomography (OCT) is a non-invasive imaging technique that produces high-resolution cross-sections of the retina. OCT is essential for detecting structural changes associated with BCD, such as retinal thinning, RPE atrophy, and the presence of crystalline deposits. OCT is particularly useful for monitoring disease progression and assessing treatment efficacy.
Fundus Autofluorescence (FAF)
Fundus Autofluorescence (FAF) imaging detects the natural fluorescence produced by lipofuscin in the RPE. This method is useful for determining and mapping the distribution of crystalline deposits and areas of retinal atrophy. FAF can help distinguish BCD from other retinal conditions that share clinical characteristics.
Electroretinography (ERG)
Electroretinography (ERG) measures the retina’s electrical responses to light stimuli. In BCD, ERG responses are typically reduced, indicating photoreceptor and RPE dysfunction. This test is especially useful for determining the functional impact of the disease on the retina.
Genetic Testing
Genetic testing is a critical part of diagnosing BCD. It entails examining a blood sample for mutations in the CYP4V2 gene. Confirming the presence of these mutations can help diagnose BCD and provide genetic counseling to affected families.
Indocyanine green angiography (ICGA)
Indocyanine Green Angiography (ICGA) is used to visualize the choroidal vasculature. In BCD, ICGA can reveal areas of choroidal sclerosis and atrophy, providing more information about the disease’s severity.
Bietti’s Crystalline Dystrophy Therapy
Currently, there is no cure for Bietti’s crystalline dystrophy, so treatment focuses on symptom management and vision preservation. A variety of approaches are used to address the disease’s complications.
Monitoring and Observation
In the early stages of BCD, when visual acuity is still relatively intact, the primary approach may be regular monitoring and observation. Patients should have regular eye exams to monitor the progression of the disease and detect any changes in the retina.
Low-Vision Aids
Low vision aids, such as magnifying glasses, large-print reading materials, and electronic reading devices, can assist patients with reduced visual acuity in maintaining independence and improving their quality of life. Occupational therapy and vision rehabilitation programs can offer additional assistance and training in the use of these aids.
Antioxidant Therapy
Although not specific to BCD, antioxidant therapy may help to reduce oxidative stress in the retina. Vitamins A, C, and E, as well as minerals like zinc and selenium, have antioxidant properties that can help maintain retinal health. Patients are frequently advised to eat a diet high in these nutrients or to take supplements as prescribed by their healthcare provider.
Genetic Counseling
Families with BCD should seek genetic counseling to better understand the disease’s inheritance pattern and implications. Counseling can inform patients about the risk of passing the condition on to their offspring and discuss genetic testing options.
Innovative and Emerging Therapies
Genetic Therapy
Gene therapy is a new treatment option that shows promise for correcting the underlying genetic defect in BCD. Researchers are looking into ways to deliver a functional copy of the CYP4V2 gene to retinal cells, which could potentially restore normal function and prevent disease progression. While still in the experimental stage, gene therapy provides hope for a future cure.
Stem Cell Therapy
Stem cell therapy is another novel approach being studied for the treatment of retinal degeneration. Researchers hope to restore vision by transplanting healthy retinal cells derived from stem cells into damaged retinas. Early-stage clinical trials are currently underway to determine the safety and efficacy of this approach in BCD.
Pharmaceutical Interventions
Pharmacological interventions aimed at the underlying mechanisms of BCD are currently being investigated. Drugs that can regulate lipid metabolism and reduce the formation of crystalline deposits are being investigated as potential treatments. These treatments are intended to slow the progression of the disease and preserve visual function.
Antioxidants and Anti-inflammatories
Antioxidant and anti-inflammatory agents are being studied for their ability to lower oxidative stress and inflammation in the retina. These treatments could help protect retinal cells from damage and slow the progression of BCD.
Retinal implants
Retinal implants, or bionic eyes, are being developed to restore vision in people with severe retinal degeneration. These devices operate by converting visual data into electrical signals that stimulate the remaining retinal cells. While still experimental, retinal implants could be a future treatment option for BCD patients with advanced vision loss.
Trusted Resources
Books
- “Inherited Retinal Disease: Diagnosis and Management” by Stephen H. Tsang
- “Retinal Dystrophies: Functional Genomics to Gene Therapy” by Gregory S. Hageman
- “Genetic Diseases of the Eye” by Elias I. Traboulsi
Online Resources
- National Eye Institute: https://www.nei.nih.gov
- American Academy of Ophthalmology: https://www.aao.org
- Foundation Fighting Blindness: https://www.fightingblindness.org
- Orphanet: https://www.orpha.net