What is hereditary retinal dystrophies?
Hereditary retinal dystrophies are a group of genetic disorders that cause progressive retinal degeneration, resulting in vision loss. These conditions are caused by mutations in the genes that control retinal cell development and function. Symptoms of hereditary retinal dystrophies include night blindness, loss of peripheral vision, and eventual central vision impairment. The severity and progression of these conditions are highly variable, depending on the specific genetic mutation involved. Early detection and genetic counseling are critical for managing these disorders and providing assistance to affected individuals and families.
In-Depth Look at Retinal Dystrophies
Hereditary retinal dystrophies include a wide range of genetic disorders affecting the retina, the light-sensitive tissue at the back of the eye that converts light into neural signals. Depending on the genetic mutation, these conditions are typically inherited as autosomal dominant, autosomal recessive, or X-linked. The most well-known hereditary retinal dystrophies are retinitis pigmentosa, Leber congenital amaurosis, Stargardt disease, and Best disease. Each of these conditions has unique genetic and clinical characteristics, but all result in progressive vision loss.
Retinitis Pigmentosa (RP
Retinitis pigmentosa is one of the most common hereditary retinal dystrophies, affecting roughly one in every 4,000 people worldwide. RP is distinguished by the progressive loss of photoreceptor cells, particularly rod cells, which are responsible for vision in low-light environments.
Pathophysiology
Mutations in over 60 genes have been linked to RP, affecting different aspects of photoreceptor function and survival. These mutations cause gradual degeneration of rod cells, followed by cone cells, which are responsible for central and color vision. Progressive photoreceptor loss causes night blindness and peripheral vision loss.
Clinical Manifestations
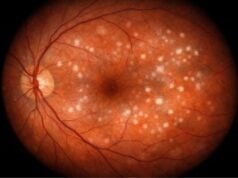
Patients with RP usually begin with night blindness in childhood or adolescence, followed by progressive loss of peripheral vision. As the disease progresses, central vision deteriorates, resulting in tunnel vision and, in some cases, complete blindness. The fundus examination reveals typical pigmentary changes, attenuated retinal vessels, and optic disc pallor.
Leber Congenital Amaurosis(LCA)
Leber congenital amaurosis is a severe form of retinal dystrophy that begins in infancy. It is one of the earliest and most severe retinal degenerations, affecting about one in every 30,000 newborns.
Pathophysiology
Mutations in at least 20 different genes cause LCA, with many of them involved in photoreceptor cell development and function, as well as the retinal pigment epithelium. These genetic defects cause widespread retinal dysfunction and degeneration from birth.
Clinical Manifestations
Infants with LCA frequently exhibit profound visual impairment, nystagmus (involuntary eye movements), and sluggish or absent pupillary responses. Electroretinography (ERG) reveals markedly reduced or absent retinal activity. Over time, affected people may develop additional ocular abnormalities like cataracts and keratoconus.
Stargardt disease
Stargardt disease is the most common type of inherited macular degeneration, affecting roughly one in every 10,000 people. It usually appears in childhood or adolescence and results in progressive central vision loss.
Pathophysiology
Stargardt disease is caused primarily by mutations in the ABCA4 gene, which codes for a protein involved in the visual cycle. These mutations cause toxic byproducts to accumulate in the retinal pigment epithelium, resulting in macula degeneration, which is responsible for sharp, central vision.
Clinical Manifestations
Patients with Stargardt disease often have difficulty reading and recognizing faces due to central vision loss. Fundus examination reveals distinctive yellowish flecks in the macula and surrounding retina. Over time, these flecks coalesce, causing macula atrophy and additional central vision loss.
Best Disease
Best disease, also known as vitelliform macular dystrophy, is an autosomal dominant condition marked by the buildup of lipofuscin-like material in the macula. It typically begins in childhood or adolescence and progresses slowly.
Pathophysiology
Best disease is caused by mutations in the BEST1 gene, which encodes the protein bestrophin-1. Although the exact function of bestrophin-1 is unknown, it is thought to regulate ion transport in the retinal pigment epithelium.
Clinical Manifestations
Patients with Best disease typically have a “egg-yolk” lesion in the macula that is visible on fundus examination. This lesion is an accumulation of lipofuscin-like material. Over time, the lesion may break down, resulting in macula atrophy and progressive central vision loss. However, Best disease progresses slowly, and many patients maintain good vision into adulthood.
Gene Basis and Inheritance Patterns
Hereditary retinal dystrophies have a variety of inheritance patterns, including autosomal dominant, autosomal recessive, and X-linked inheritance. Understanding these patterns is critical for genetic counseling and assessing risk in affected families.
- Autosomal Dominant Inheritance: A single copy of the mutated gene passed down from one parent is enough to cause the disorder. Conditions such as Best disease and some types of RP follow this pattern.
- Autosomal Recessive Inheritance: The disorder can only be caused by two copies of the mutated gene, one from each parent. LCA and Stargardt disease frequently exhibit this pattern.
- X-Linked Inheritance: The mutant gene is found on the X chromosome. Males are more severely affected, whereas females can be carriers with milder symptoms. Some types of RP are X-linked.
Effects on Quality of Life
Hereditary retinal dystrophies have a significant impact on quality of life because vision loss progresses over time. Patients frequently encounter difficulties in education, employment, and daily living activities. The psychological effects can be severe, resulting in anxiety, depression, and social isolation. Early detection, genetic counseling, and assistance from healthcare providers and support groups are critical for overcoming these obstacles.
Diagnostic Approaches for Retinal Dystrophies
Hereditary retinal dystrophies are diagnosed using a combination of clinical evaluation, imaging studies, and genetic tests. Early and accurate diagnosis is critical for effective treatment and genetic counseling.
Clinical Evaluation
A thorough clinical examination is the first step in diagnosing hereditary retinal dystrophies. This includes:
- Patient History: Detailed documentation of the onset, progression, and nature of visual symptoms, as well as a family history of related conditions.
- Visual Acuity Testing: Measuring central and peripheral vision to determine the degree of vision loss.
- Ophthalmoscopic Examination: Examine the retina for distinctive findings such as pigmentary changes, retinal flecks, or macular lesions.
Imaging Studies
Imaging studies are critical for visualizing retinal structures and determining the extent of degeneration:
- Optical Coherence Tomography (OCT): Produces high-resolution cross-sectional images of the retina, allowing for detailed examination of retinal layers and the detection of structural abnormalities.
- Fundus Photography: Produces detailed images of the retina, allowing for the documentation of characteristic findings and the monitoring of disease progression.
- Fluorescein Angiography: This procedure involves injecting a fluorescent dye into the bloodstream to visualize retinal blood vessels and detect abnormalities.
Genetic Testing
Genetic testing is the definitive method for diagnosing hereditary retinal dystrophies, which involves analyzing DNA samples to identify specific mutations associated with the condition. This includes:
- Targeted Gene Panels: Look for a specific set of genes linked to retinal dystrophies.
- Whole Exome Sequencing (WES): Analyzing all genes’ coding regions for mutations.
- Whole Genome Sequencing (WGS): Examining the entire genome for genetic variations.
Electrophysiological Tests
Electrophysiological tests can provide more information about retinal function.
- Electroretinography (ERG): Measures the retina’s electrical responses to light stimuli, which aids in determining photoreceptor functionality.
- Visual Evoked Potentials (VEP): Measures the electrical activity of the brain in response to visual stimuli, which aids in determining the functional integrity of the optic pathways.
Treatment Options for Retinal Dystrophies
The treatment of hereditary retinal dystrophies focuses on symptom management, slowing disease progression, and improving patients’ quality of life. While there is no cure for these conditions, several therapeutic approaches and emerging treatments provide hope.
Standard Treatment Options:
- Nutritional Supplements: Antioxidants like vitamin A, E, and omega-3 fatty acids may slow the progression of some retinal dystrophies. High-dose vitamin A palmitate has been shown to slow the progression of retinitis pigmentosa in some patients.
- Low Vision Aids: Magnifiers, telescopic lenses, and specialized lighting can improve remaining vision, allowing patients to complete daily tasks and maintain independence.
- Assistive Technologies: Screen readers, braille displays, and voice-activated devices can improve access to information and communication, significantly improving the quality of life for people suffering from severe vision loss.
- Genetic Counseling: Informing affected individuals and their families about the inheritance patterns and implications of hereditary retinal dystrophies allows them to make more informed decisions about family planning and condition management.
Innovative and Emerging Therapies
- Gene Therapy: Gene therapy seeks to correct the underlying genetic defect that causes retinal dystrophy. Luxturna (voretigene neparvovec-rzyl) is the first FDA-approved gene therapy to treat Leber congenital amaurosis caused by RPE65 mutations. This treatment involves injecting a normal copy of the RPE65 gene directly into the retina, which restores some vision function.
- Stem Cell Therapy: Researchers are looking into the use of stem cells to replace damaged retinal cells. Induced pluripotent stem cells (iPSCs) derived from a patient’s own cells can be differentiated into retinal cells and implanted into the retina to restore function. This approach shows promise, but it is still in the experimental stage.
- Pharmacological Approaches: Several drugs are being tested to protect retinal cells from degeneration. Clinical trials are currently underway for neuroprotective agents that inhibit apoptosis (cell death) pathways or reduce oxidative stress.
- Retinal Implants: Patients with advanced retinal dystrophy can have partial vision restored with retinal prostheses such as the Argus II retinal implant. These devices convert visual information into electrical signals, which stimulate the remaining retinal cells, resulting in artificial vision.
- CRISPR/Cas9 Gene Editing: This ground-breaking technology allows for precise editing of DNA to correct genetic mutations. Researchers are looking into using CRISPR/Cas9 to treat different types of hereditary retinal dystrophy by repairing the defective genes that cause the condition.
Clinical trials
Participating in clinical trials gives patients access to cutting-edge therapies while also helping to advance our understanding of these conditions. Patients with hereditary retinal dystrophies should speak with their doctors about current clinical trials and the potential benefits of participating.
Combining these treatment strategies can help healthcare providers better manage hereditary retinal dystrophies, slowing disease progression and improving affected individuals’ quality of life.
Best Practices to Prevent Hereditary Retinal Dystrophies
While hereditary retinal dystrophies are genetic and cannot be completely avoided, certain practices can help manage the condition and potentially reduce its impact.
- Genetic Counseling: People with a family history of hereditary retinal dystrophies should get genetic counseling to better understand their risk and consider genetic testing. Early detection enables timely intervention and management.
- Regular Eye Exams: Routine eye exams can detect early signs of retinal dystrophy. Individuals at risk should have comprehensive eye exams once a year, or as recommended by their ophthalmologist.
- Healthy Diet: Eating a well-balanced diet high in antioxidants, vitamins, and omega-3 fatty acids can benefit overall eye health. Leafy greens, fish, nuts, and fruits are especially nutritious.
- Protect Eyes from UV Light: Wearing UV-blocking sunglasses can help protect the retina from damage caused by sunlight.
- Avoid Smoking: Smoking increases oxidative stress and hastens retinal degeneration. Quitting smoking can improve overall eye health.
- Manage Systemic Health Conditions: Properly managing conditions like diabetes and hypertension can reduce the risk of secondary complications that can harm retinal health.
- Stay Informed: Staying up to date on the latest research and developments in the field of hereditary retinal dystrophies can assist patients and families in making informed decisions about their care and potential clinical trial participation.
Individuals at risk for hereditary retinal dystrophies can take proactive steps to monitor their eye health and potentially reduce the severity of these conditions by implementing these preventive measures.
Trusted Resources
Books
- “Inherited Retinal Diseases: A Diagnostic and Therapeutic Approach” by Stephen H. Tsang
- “Retinal Degenerative Diseases: Mechanisms and Experimental Therapy” by Catherine Bowes Rickman, Matthew M. LaVail, and Christine Grimm
- “Mitochondrial Dysfunction in Neurodegenerative Disorders” by Amy Katherine Reeve and Swee Hong Gee