Therapeutic hypothermia is rapidly emerging as a forward-thinking strategy to help safeguard visual function following serious eye injuries. Rather than simply masking the symptoms of damage, this cooling-based approach offers the potential for proactive tissue protection, leveraging precise temperature management to reduce inflammation and limit cellular harm.
Healthcare providers are increasingly turning to targeted temperature reduction techniques in efforts to mitigate the long-term effects of ocular trauma. Through a process that cools damaged eye structures and surrounding tissues, therapeutic hypothermia may preserve or even improve outcomes, offering a glimmer of hope in situations where conventional treatments alone have fallen short.
Putting Cooling Solutions in Perspective
Therapeutic hypothermia has roots in established medical procedures that use controlled cooling to protect injured or at-risk tissues. Initially recognized for its benefits in cases like cardiac arrest or severe head trauma, it is now gaining traction within ocular healthcare. The principle is straightforward: by lowering tissue temperature, cell metabolism slows down, which can stave off or reduce the severity of secondary damage that often follows acute injury. When the eye sustains trauma—be it from a blunt-force impact, sharp laceration, or chemical burn—there is a narrow window in which to minimize inflammation, hemorrhage, and swelling. During that window, carefully induced cooling may help prevent irreparable harm.
The biology behind this method is intricate but fascinating. At the core, when cells experience a damaging event, a cascade of biochemical reactions occurs, often speeding up processes that lead to further injury. Therapeutic hypothermia intervenes by lowering the overall metabolic rate of these cells, effectively slowing or halting the chain reactions that produce destructive free radicals and inflammatory markers. This gives the eye’s natural repair processes a better chance to function without overwhelming stress. Although this concept is promising, it remains crucial to remember that cooling therapy alone is typically just one component of a broader treatment plan, which may include medication, surgery, or other supportive measures to fully address the type and extent of ocular damage.
A Novel Direction for Eye Care
In an era of increasing demand for innovative healthcare solutions, therapeutic hypothermia stands out as a technique that marries cutting-edge physiology with a user-friendly delivery process. Medical professionals have long recognized the value of controlling inflammation after injuries. However, cooling therapy offers a targeted, localized way to achieve that control around the eye, which is especially beneficial for patients with injuries that threaten their visual acuity. By honing in on a physical parameter—temperature—the approach bypasses many of the side effects associated with other interventions like high-dose medications.
The level of customization involved in cooling therapy also aligns well with modern patient-centric care. Because every ocular injury is unique, eye specialists can tailor the intensity and duration of cooling to match individual needs. A more severe trauma, for example, might warrant a longer or deeper hypothermic cycle to reduce the risk of tissue death, while minor injuries may only need a brief cooling protocol. This kind of tailoring not only enhances the chances of success but also mitigates complications that might arise if a uniform approach were used for all cases.
Where Traditional Methods Fall Short
Historically, ocular trauma management has relied heavily on surgical interventions, antibiotics, steroids, or other systemically active drugs to handle inflammation and infection. While these strategies can be effective in many respects, they often come with a host of side effects or limitations. Systemic medications, for instance, might compromise other organs or systems in the body. Local therapies, such as eye drops or ointments, are typically designed to focus on surface-level issues like corneal abrasions or mild infections; they may not adequately address deeper-level trauma in the back of the eye or swelling within the vitreous cavity.
In contrast, therapeutic hypothermia treats inflammation and tissue injury at the cellular level, targeting the metabolic processes that lead to edema, scarring, or even total vision loss. By cooling the tissues, blood flow can be modulated in such a way that swelling and further hemorrhage may be minimized. Although this therapy may not eliminate the need for surgical correction in severe cases, it can serve as a valuable adjunct, potentially making recovery smoother and less traumatic overall.
A Growing Global Consensus
Worldwide, more and more ophthalmology departments and trauma centers are evaluating the merits of cooling therapy for eye injuries. Factors such as improved technology, greater availability of specialized cooling devices, and robust clinical evidence in related medical fields (e.g., neurology) all support this growing acceptance. While the approach still has its skeptics—particularly those who argue it may not be fully standardized—an increasing body of anecdotal and research-based outcomes indicates that the right combination of cold application and adjunct therapies can significantly enhance patient prognoses.
Additionally, the shift toward therapeutic hypothermia in eye care highlights a larger trend in medicine that leans toward minimally invasive yet highly targeted interventions. As the global population ages and the potential for eye injuries due to accidents or degenerative conditions increases, the need for advanced therapies that improve outcomes and reduce costs simultaneously has never been greater. Cooling therapy sits squarely at the intersection of innovation and practicality, offering a tangible route to enhanced vision preservation.
Potential Limits and Ongoing Debates
Despite its promise, therapeutic hypothermia is not without complexities. Eye specialists must consider how to precisely regulate temperature, how quickly to induce cooling, and how to balance the therapy with other necessary interventions—particularly in cases where a patient’s blood pressure or heart rate might be unstable from other injuries. Overly aggressive or improperly monitored cooling could risk damaging healthy tissues or slowing vital processes needed for healing.
There is also the matter of timing. For optimal results, cooling therapy should begin as quickly as possible after an injury. This narrow treatment window requires medical teams to be well-prepared, with readily available cooling equipment and protocols. As a result, this approach is often most feasible in specialized centers where ocular trauma is frequently treated. Still, as awareness grows, the infrastructure for this form of therapy is likely to expand, making it more widely accessible over time.
Integrating Innovation with Clinical Wisdom
One of the major appeals of therapeutic hypothermia is its synergy with existing medical knowledge about trauma management. When doctors combine cooling therapy with established interventions—like wound suturing, antibiotic administration, or anti-inflammatory drugs—they can tackle a broader range of injury complications. Rather than seeing cooling as an outright replacement for current treatments, many vision experts now view it as an essential component of a robust, multi-pronged approach. The result can be a more holistic care plan that addresses both immediate damage and secondary complications that could threaten sight in the long run.
Equally important is patient and family education. Because therapeutic hypothermia is still relatively new in the field of ophthalmology, people may be hesitant or uncertain about its benefits. Clear communication from healthcare providers—explaining how the therapy works, its potential outcomes, and the short-term sacrifices (like temporary discomfort from cold application)—helps encourage patient compliance and engagement. In addition, understanding the rationale behind cooling can alleviate anxiety, positioning the therapy not as an experimental tactic but as a strategic measure with real, measurable benefits.
Shaping the Future of Eye Trauma Interventions
From multi-specialty trauma centers to dedicated ophthalmology clinics, therapeutic hypothermia is steadily earning its place as a potent strategy for preserving eyesight. By merging a meticulous understanding of ocular physiology with advancements in medical technology, this technique offers real hope for individuals who experience severe eye injuries. In the coming years, further research is expected to refine guidelines for how and when cooling therapy should be used, making it even more effective and accessible. Consequently, patients and medical professionals alike stand to benefit from a therapy that not only promises improved outcomes but also aligns with broader trends toward minimally invasive, precise, and patient-oriented care.
Applying Targeted Temperature Reduction for Eye Injuries
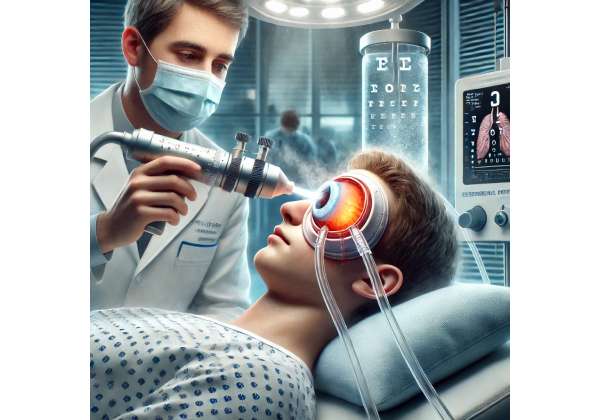
Therapeutic hypothermia for ocular trauma, at its core, revolves around carefully cooling the affected eye and its surrounding tissues to mitigate harmful processes that typically accompany severe injury. Although the concept may sound simple, its execution requires meticulous planning to ensure effectiveness. In many cases, specialized cooling devices such as water-circulating cooling masks or localized cryotherapy systems are employed to deliver consistent, targeted temperature reduction directly to the region of concern.
Preparing the Patient and Setting
When a patient with ocular trauma arrives at a medical facility, the immediate priority is to conduct a rapid but thorough assessment of the overall injury. This often includes imaging techniques like ultrasound or CT scans, alongside a direct evaluation of eye function—checking for pupil response, visual acuity, and potential structural damage. Once medical professionals determine that the patient could benefit from cooling therapy, they outline a clear protocol that delineates the specific temperature range, duration of cooling, and any accompanying treatments.
Patients and caregivers should be informed about what to expect, including how long the therapy might last and the sensations—such as mild discomfort or numbness—that can occur from the cooling process. If other procedures, such as suturing or administration of antibiotics, are also necessary, those steps are usually taken either before or at carefully timed intervals during the cooling period to ensure no interference with the therapy’s objectives.
Key Steps in the Cooling Protocol
- Temperature Selection: Medical teams identify a narrow temperature range that has been proven to optimize tissue preservation without leading to hypothermic complications. This range often falls a few degrees below normal body temperature, usually in the mid-to-lower 30 degrees Celsius range, although exact numbers may vary based on individual cases and institutional guidelines.
- Application Method: Cooling may be delivered via specialized equipment. Some devices wrap around the head and maintain steady cooling, while others resemble goggles or masks that provide localized chill directly to the orbital area. In more resource-limited settings, simpler methods like chilled saline compresses might be employed, although these can be harder to regulate.
- Monitoring: Throughout the therapy, clinicians keep a close eye on both the eye tissue temperature and the patient’s general vital signs. Nurses or trained staff check for excessive cold exposure, potential frostbite, or signs that the therapy is becoming uncomfortable or unsafe.
- Rewarming Phase: After a set duration—commonly ranging from 30 minutes to several hours, depending on the severity of the injury—a gradual rewarming process begins. Abrupt shifts in temperature can cause additional harm, so this step is carried out slowly, returning the eye to normal body temperature under controlled conditions.
Timing and Duration
The exact timing of cooling therapy often depends on the nature of the trauma. If the injury is very recent (within minutes to an hour), the chances of preventing secondary damage are substantially higher, making immediate cooling a top priority. In many protocols, clinicians aim to begin temperature reduction as soon as the patient is stabilized. Duration can differ widely—from short 20-minute intervals for mild injuries to prolonged cooling sessions for severe cases. The overarching principle is that cooling should last only as long as it continues to yield protective effects and should not be extended to the point where it compromises blood flow or normal tissue metabolism in the eye.
Synergizing with Other Treatments
Therapeutic hypothermia rarely operates in isolation. Instead, it often runs in tandem with other interventions. For instance, if an ocular injury involves open wounds, stitches might be necessary to seal potential entry points for infection. Steroids could be administered systemically or locally to manage excessive inflammation, while antibiotics guard against bacterial complications. Cooling therapy complements these treatments by lowering the overall metabolic demand in the injured tissue, effectively giving the eye a better environment in which to heal.
Moreover, in cases of severe trauma, the use of artificial tears or lubricating ointments can help maintain moisture on the eye’s surface, which is critical if the blink reflex is compromised or if the patient is under sedation. This all-hands-on-deck approach underscores how integrated care can significantly boost the patient’s chance of retaining useful vision.
Addressing Discomfort and Anxiety
Maintaining patient comfort is crucial, especially when dealing with the sensitive structure of the eye. Many individuals are apprehensive about the sensation of cold being applied directly near or around their eyes. Healthcare providers often use mild anesthetics to ease any pain or irritation. Additionally, reassuring communication about how the therapy progresses and what sensations to expect can alleviate anxiety. In pediatric cases, where cooperation might be limited, sedation or general anesthesia may be considered for short durations to ensure both safety and efficacy.
Adapting Protocols for Special Populations
Not all patients are good candidates for therapeutic hypothermia. Those who have certain circulatory or cardiovascular issues, for example, might be at increased risk if exposed to localized cooling. Elderly patients or individuals with compromised immune systems also need additional monitoring to avoid complications, such as reduced wound healing capacity. In these contexts, the standard cooling times or temperature ranges might need to be adjusted. The principle of “start low, go slow” can guide medical professionals, meaning they apply a more conservative temperature reduction initially and evaluate the patient’s response before proceeding to deeper cooling.
The Role of Follow-Up Visits
The effects of therapeutic hypothermia for ocular injuries extend well beyond the period of active cooling. Subsequent check-ups allow medical teams to gauge the success of the intervention and watch out for late-emerging issues like infections or chronic inflammation. During these visits, ophthalmologists assess the healing progression of the eye, often using detailed imaging tools to track internal structures for signs of scarring, fluid buildup, or vascular changes. Additional treatments can be prescribed as necessary, and further cooling sessions might be recommended in rare situations where swelling reappears.
Streamlining Protocols Over Time
As the application of therapeutic hypothermia gains traction in ophthalmology, expect protocols to evolve based on clinical outcomes, research findings, and technological improvements. Devices are becoming more precise, offering software-guided temperature regulation that automatically adjusts cooling levels. These advancements, coupled with accumulating data on optimal durations, best temperature thresholds, and patient selection criteria, will undoubtedly refine how healthcare providers implement this therapy. Ultimately, the goal remains the same: to ensure that every patient with ocular trauma has the best possible odds of retaining functional, healthy vision.
Up-to-Date Findings and Clinical Trials
A growing body of evidence supports therapeutic hypothermia as a promising tool for preserving eye health after serious injuries. Although historically more research has focused on cooling therapy for conditions like cardiac arrest and neonatal encephalopathy, recent studies have begun to examine its impact on ocular structures. These investigations have provided key insights into not only how cooling affects different layers of the eye—from the cornea to the retina—but also the best practices for timing and intensity of temperature reduction.
Pioneering Investigations
One of the earliest documented studies analyzing localized hypothermia for ocular injuries took place within a small cohort of patients who had sustained corneal lacerations. Published several years ago in an international ophthalmology journal, this preliminary research reported that individuals receiving controlled cooling showed lower rates of infection and a reduced need for post-operative steroid therapy. Though the study population was limited, its findings laid the groundwork for larger, more systematic investigations.
Further exploration revealed that cooling therapy might be particularly helpful when administered within a tight time window after traumatic events, reinforcing the commonly held view that early intervention plays a pivotal role. Some research even indicates that immediate cooling can stem the flow of destructive enzymes and inflammatory mediators, giving the eye valuable time to recover. These beneficial effects were also observed in advanced imaging scans, which showed reduced edema in patients who underwent therapeutic hypothermia protocols.
Larger-Scale Trials and Meta-Analyses
Over the past decade, more robust clinical trials have emerged, featuring bigger patient pools and randomized study designs. A notable multi-center study published in a prominent ophthalmic journal in 2018 highlighted the therapy’s potential for patients with severe eye trauma. Among the findings, participants who received well-monitored cooling exhibited better visual outcomes and a lower incidence of complications such as retinal detachment compared to the control group receiving standard care.
Subsequent meta-analyses, reviewing data from multiple independent studies, have largely echoed these positive trends. While the exact magnitude of benefit varies—some trials indicate small to moderate improvements, whereas others suggest more pronounced changes—there is general consensus that cooling therapy contributes to diminished inflammation and scarring. However, these collective reviews also underline the need for better standardization of cooling procedures, as differences in device types, temperature targets, and treatment durations can significantly impact results.
Novel Therapies and Combination Approaches
Researchers are increasingly looking at therapeutic hypothermia not as a standalone method but as one piece of a broader puzzle. In some forward-thinking protocols, cooling is paired with specialized drugs that inhibit angiogenesis or inflammation. One proof-of-concept study, conducted by a group of ophthalmologists at a leading research hospital, showed that combining mild hypothermia with a topical anti-inflammatory agent led to enhanced corneal healing in patients with penetrating injuries.
Other investigations have centered on the synergy between cooling and surgical interventions. For instance, in cases of ocular fractures or severe intraocular bleeding, surgeons may operate to stabilize the eye’s structure, then follow up with a targeted cooling protocol. Preliminary data suggests that this dual-stage approach might result in faster healing and a reduced risk of scarring compared to surgery alone, although more controlled trials are necessary to determine definitive guidelines.
Case Reports and Real-World Examples
Beyond controlled trials, case reports offer valuable insights into how therapeutic hypothermia functions in real-world situations. Several documented cases describe patients with acute chemical burns to the cornea who experienced significantly less ulceration and infection after receiving immediate localized cooling. In these situations, the therapy effectively counteracted the chemical agents while also moderating the inflammatory response. Stories of individuals walking away with near-normal vision after potentially blinding injuries serve as powerful anecdotal evidence supporting the therapy’s utility.
Field hospitals in some regions, where ocular trauma is more prevalent due to accidents or conflict situations, have also adopted makeshift cooling methods. Although less precise than hospital-grade equipment, these improvised solutions use chilled fluids or cold packs to stabilize the eye until the patient can reach a more advanced care setting. While controlled research on such real-world applications remains sparse, the anecdotal success further underlines the adaptability and life-changing potential of cooling therapy.
Research Gaps and Ongoing Studies
Despite the encouraging findings, many questions remain. There is significant interest in clarifying the biological mechanisms that underlie hypothermia’s protective role, particularly at the cellular and molecular levels. For instance, some scientists are investigating whether cooling might upregulate certain genes tied to tissue regeneration or downregulate those associated with cell death. Others aim to identify whether certain subgroups of patients—such as the elderly, diabetics, or individuals with pre-existing ocular conditions—benefit more or less from the therapy than the general population.
Concurrent investigations focus on refining the technical aspects of cooling, such as how quickly to lower temperature, how to maintain it at a stable level, and how long to continue the therapy before rewarming. Smaller pilot studies have tested various cooling durations, comparing short bursts of intense cold with more moderate temperature drops sustained over longer periods. These nuanced variations can make a difference in outcomes, further illustrating that a one-size-fits-all approach may not be appropriate.
Broader Adoption and Education
As the knowledge base expands, educational initiatives are also gaining ground. In some training programs, ophthalmology residents now learn about therapeutic hypothermia as part of their curriculum, including how to identify suitable candidates and implement best practices for temperature management. Simultaneously, professional conferences have dedicated sessions to highlight new data and explore the complexities of applying this therapy in diverse clinical scenarios.
The widespread acceptance of therapeutic hypothermia will likely hinge on these continued efforts to validate its efficacy and standardize its use. Expanding research initiatives, documented best practices, and robust outcome data all contribute to building confidence in the method’s safety and efficacy. Over time, these strides could transform therapeutic hypothermia from a somewhat niche approach into a recognized pillar of ocular trauma care.
Future Directions to Watch
In the coming years, expect more sophisticated cooling devices designed specifically for ocular use. Enhanced tools may feature built-in feedback mechanisms that automatically adjust temperature based on real-time tissue data. This kind of precision could reduce the risk of overcooling and improve patient comfort. Furthermore, the development of specialized cooling gels or patches that can be applied swiftly in emergency settings could make the therapy more accessible to patients worldwide, especially in regions lacking comprehensive medical infrastructure.
Researchers are also examining whether cooling technology might have applicability beyond acute trauma. For instance, certain degenerative eye conditions or inflammatory disorders might benefit from short-term hypothermia treatments to curb damaging flare-ups. While these newer uses remain in exploratory phases, the concept opens the door to a host of possibilities that could expand the role of cooling therapy in eye care.
Assessing Real-World Impact and Potential Adverse Events
Therapeutic hypothermia has proven impactful in reducing the severity of ocular trauma and promoting better post-injury recovery. Many individuals report less pain, clearer vision, and faster return to daily activities when cooling therapy is administered early and in conjunction with other medical interventions. Studies suggest that tissue preservation from immediate cooling may help reduce the necessity for invasive follow-up procedures, further highlighting its value in real-world scenarios.
Yet as with any medical intervention, potential risks exist. Common complaints include localized discomfort or a sensation of numbness in the treated area. In rarer instances, overcooling can lead to frostbite-like symptoms or delayed wound healing if not carefully monitored. There is also a small risk of creating a more pronounced immunosuppressive effect in the ocular tissues, making infection control protocols even more essential. By working closely with an experienced healthcare team, most patients find these concerns manageable, and serious complications remain relatively rare.
Cost Analysis for Cooling Therapy Access
The cost of implementing therapeutic hypothermia for eye injuries can vary, influenced by factors like the severity of trauma, the facility’s equipment, and insurance coverage. Pricing may range from moderate fees for basic cooling techniques to higher charges for advanced, device-driven procedures. Some health plans reimburse a significant portion, especially if the therapy is deemed medically necessary. Patients are encouraged to check with their insurance providers and consult hospital billing departments to confirm the most recent figures for their specific situation.
This article is provided for educational purposes only and is not a substitute for professional medical guidance. Always consult a qualified healthcare provider for personalized advice. If you found this information valuable, consider sharing it on Facebook, X, or any platform you use, helping others discover the potential of therapeutic hypothermia for ocular trauma.