Glaucoma is one of the most common causes of irreversible blindness worldwide, largely due to its progressive nature and frequent lack of early warning signs. Although many individuals manage intraocular pressure (IOP) with topical drops, compliance can be a struggle. Busy schedules, forgetfulness, and discomfort from repeated eye-drop application all contribute to inconsistent usage, ultimately placing the optic nerve at greater risk. This precarious balancing act has driven researchers to explore more reliable methods that ensure stable drug delivery, paving the way for innovations designed to simplify the patient experience.
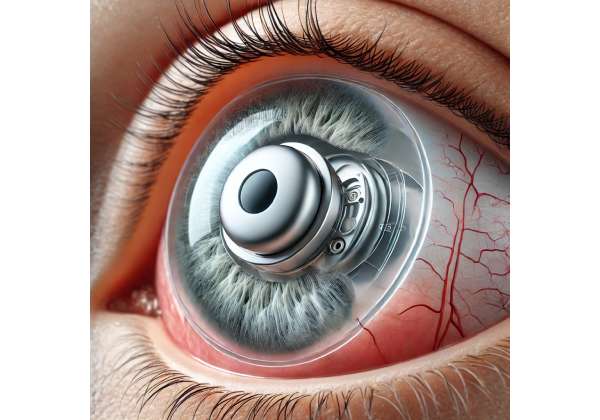
Among these breakthroughs stands Glaukos iDose®, a novel sustained-release implant that delivers medication to the eye continuously over extended periods. This miniature device sits securely within the eye’s anterior chamber, slowly releasing a stable dose of intraocular pressure-lowering medication. By shifting away from daily drops and complicated regimens, iDose promises to reduce noncompliance, allowing patients to focus less on routine reminders and more on living confidently. In the sections that follow, we will explore how iDose works, review the protocols for its implantation, delve into current scientific data, and evaluate the safety profile and costs associated with this transformative approach to glaucoma management.
Why iDose Sustained Release Is Changing Glaucoma Care
iDose is designed with a singular purpose in mind: to simplify glaucoma management by providing a steady stream of medication without the need for frequent patient intervention. Traditional glaucoma therapies often require multiple daily instillations of eye drops—ranging from prostaglandin analogs to beta-blockers—to keep intraocular pressure within a safe range. When these therapies are used inconsistently, episodes of elevated pressure can inflict further damage on the optic nerve, leading to progressive vision loss. iDose breaks this cycle by offering sustained, low-level drug release.
A Revolutionary Design for Long-Term Efficacy
At its core, the iDose implant is a small titanium reservoir filled with an intraocular pressure-lowering agent, typically a prostaglandin analog. The reservoir is engineered to release this agent at a controlled rate, ensuring that patients maintain consistent therapeutic levels over an extended timeframe—potentially months or even years before a replacement or refill is necessary. This addresses a longstanding issue in glaucoma care: balancing the need for potent medication with the reality that humans struggle to adhere to daily regimens over the long term.
From a design standpoint, iDose leverages microtechnology to remain unobtrusive. Though small in size, it is robust enough to withstand normal ocular movements and changes in daily activity. Once placed in the eye, the device is anchored such that it does not float or move around, minimizing irritation. Through a specially designed membrane or valve mechanism, the drug molecules diffuse at a calibrated rate, bypassing fluctuations that can occur with traditional daily drops.
Shifting the Patient Experience
For many individuals, the initial diagnosis of glaucoma is accompanied by concerns over vision loss and the responsibility of treatment adherence. Eye drops, while effective, can be uncomfortable, and forgetting even a few doses per week can compromise outcomes. iDose alleviates much of this burden by handing off the responsibility of medication delivery to a well-engineered implant. Rather than multiple drops per day, patients may only need periodic monitoring to confirm the device’s efficacy, reducing stress and streamlining care.
This approach holds particular promise for patients struggling with manual dexterity, such as those with arthritis or tremors, who often find eye-drop application challenging. It can also be a game-changer for individuals with cognitive impairments or those managing multiple health conditions. By internalizing drug administration, iDose may drastically cut down on user error, potentially stabilizing eye pressures more effectively than patchy self-administration of drops.
Expanding Possibilities in Glaucoma Management
iDose is part of a broader trend in micro-invasive glaucoma surgery (MIGS) and sustained-release treatments that aim to minimize the side effects of repeated topical exposure—like ocular surface irritation—while maximizing drug effectiveness. By localizing the medication within the eye, the therapy sidesteps many systemic absorption issues associated with topical or oral drugs.
In addition, because iDose consistently releases medication, clinicians can more confidently assess how a patient’s eye pressure responds to a known, stable drug concentration. When used in conjunction with other glaucoma interventions (like MIGS devices for improved trabecular outflow or cyclophotocoagulation procedures), iDose may offer a multi-pronged strategy that spans both mechanical and pharmacological interventions.
Ultimately, iDose stands poised to reshape how patients and healthcare providers view the day-to-day realities of glaucoma care. With a single procedure, it is possible to ensure a steady supply of medication, freeing both the patient and ophthalmologist from the potential pitfalls of noncompliance or inconsistent dosing. In the subsequent sections, we will look more closely at how this device is placed, the protocols surrounding it, and the evidence supporting its use.
Placing the Device: Step-by-Step Protocol
Successful outcomes with iDose hinge on proper device placement and a clear understanding of its application. Because iDose remains in situ for an extended duration, a meticulous surgical or procedural approach is vital to minimize complications and ensure optimal positioning. Below is an overview of how ophthalmologists typically administer iDose, from preoperative planning to post-insertion follow-up.
Preoperative Evaluations and Planning
Before scheduling an iDose procedure, patients typically undergo a thorough ophthalmic examination. This includes:
- Measuring Intraocular Pressure (IOP): Establishing a baseline helps track changes post-implant.
- Optic Nerve Imaging: Utilizing optical coherence tomography (OCT) or other imaging techniques to assess optic nerve health and rule out advanced structural damage.
- Anterior Chamber Assessment: Evaluating angle anatomy via gonioscopy to ensure that iDose can be placed securely in a position where it does not interfere with normal aqueous outflow or create internal eye trauma.
- Corneal Integrity Check: Inspecting corneal thickness and endothelial cell counts, important for any intraocular procedure, though iDose is relatively gentle in terms of corneal touch.
During these preliminary stages, the ophthalmologist also confirms the patient’s current medication regimen and evaluates any comorbidities that might influence healing (e.g., diabetes, rheumatoid conditions, or blood clotting disorders). Where needed, other preoperative measures—like adjusting certain systemic medications or providing prophylactic eye drops—may be considered to reduce the risk of infection or inflammation.
The Insertion Procedure
While the exact technique can vary depending on surgeon preference, the iDose procedure generally unfolds as follows:
- Topical or Local Anesthesia: Patients typically receive numbing eye drops or a local anesthetic injection around the eye to maintain comfort during the procedure.
- Small Incision: Using specialized microsurgical instruments, the surgeon creates a tiny self-sealing incision in the clear cornea or near the limbus. This incision is large enough to accommodate the device but small enough to ensure rapid healing and minimal postoperative issues.
- Secure Placement: The iDose implant, pre-loaded with the intraocular medication, is introduced through the incision. Surgeons use visualization tools (like a gonioscope or microscope) to anchor it in the desired angle or within the anterior chamber. The design of iDose includes a retention mechanism that helps secure it in place.
- Incision Closure: If the incision is corneal, it often self-seals. Otherwise, the surgeon may apply micro-sutures or tissue sealants. Intraocular antibiotic or anti-inflammatory solutions may be injected to reduce infection and inflammation risk.
Because iDose is compact, the procedure often resembles other micro-invasive glaucoma surgeries in terms of operating time and recovery. Many patients can undergo this as an outpatient process, experiencing relatively little discomfort or downtime.
Immediate Postoperative Care
Following implantation, most patients wear a protective shield or patch for a day or two to minimize inadvertent rubbing or pressure on the eye. The ophthalmologist usually prescribes antibiotic and anti-inflammatory drops for a short duration to assist in healing and prevent complications. Within the first week, the surgeon checks for:
- Proper Device Position: Ensuring the implant remains stable and that no migration or malpositioning has occurred.
- Intraocular Pressure Levels: Confirming that iDose is releasing sufficient medication to maintain healthy IOP ranges.
- Signs of Infection or Inflammation: Redness, pain, or discharge warrant immediate attention, though such complications are relatively rare.
Long-Term Monitoring
Post-procedure follow-ups might take place at intervals similar to those used in traditional glaucoma management—often at one month, three months, and six months, then as deemed necessary. Key aspects include:
- IOP Measurement: Intraocular pressure should remain stable. Sudden elevations could signal that the device is malfunctioning, has dislodged, or that the medication release is insufficient for the patient’s needs.
- Visual Field Tests: Periodic perimetry or other functional tests verify that the patient’s vision remains stable and that disease progression is halted.
- Device Integrity: If imaging or clinical signs raise concerns, the implant’s position and functionality may be re-evaluated. In rare instances, a second procedure might be required to adjust or remove the device.
One of the major conveniences iDose offers is the potential for extended intervals without changing medication bottles or adjusting multiple daily eye drops. Patients can often stick to routine check-ups without the worry of forgetting a dose. Over time, if the medication reservoir depletes or if the patient’s needs change, the surgeon can remove and replace the iDose with a fresh implant or consider a refill approach depending on the latest device generation.
Overall, the iDose placement protocol emphasizes efficiency, minimal invasiveness, and a smooth recovery. This stands in contrast to more extensive glaucoma surgeries like trabeculectomy or tube shunts, which often come with longer healing times and higher risk of complications. By offering a streamlined route to consistent medication delivery, iDose helps patients maintain stable pressures with fewer daily hassles—a significant leap forward for those whose main struggle lies in compliance and routine.
Clinical Evidence and Academic Findings
iDose has captured the attention of both practicing ophthalmologists and vision scientists, sparking numerous studies aimed at validating its safety and efficacy in controlling intraocular pressure. The technology sits at the intersection of controlled drug delivery and micro-invasive procedures, a convergence that has intrigued the medical community eager to reduce the burden of patient noncompliance. Below is an overview of the key findings from published data, ongoing trials, and real-world observations.
Early Feasibility Studies
Before large-scale clinical trials, Glaukos and independent investigators conducted initial feasibility and safety assessments. These smaller studies focused on confirming that the implant could:
- Consistently Release Medication: Measuring daily or weekly drug concentrations in aqueous samples or analyzing IOP trends in early volunteers.
- Remain Secure: Ensuring minimal device migration or rotation within the anterior chamber over several months.
- Evade Serious Complications: Monitoring for signs of corneal endothelial cell loss, angle damage, or unusual inflammation.
Reports from these early trials were encouraging: iDose implants stayed in place, with no significant changes in corneal health or increased rates of infection. Many participants experienced stable or lowered IOP compared to their baseline, particularly those who transitioned from a less consistent drop regimen.
Larger Pivotal Trials
Building on that foundation, phase II or III trials examined iDose in a controlled, often randomized manner, comparing it to standard-of-care topical medications. In these studies, participants were typically divided into groups—some receiving the iDose implant, others continuing with or starting on daily prostaglandin analog drops. Over six to twelve months, researchers documented:
- Change in IOP: The primary endpoint in most glaucoma studies. Participants with iDose often showed comparable or slightly better results in reducing IOP, particularly in those at risk for noncompliance.
- Number of Adverse Events: Incidences of ocular hypertension, device dislocation, or infection. Rates generally remained low and in line with other micro-invasive procedures.
- Quality of Life Measures: Tools like the Glaucoma Quality of Life questionnaire or the Ocular Surface Disease Index sometimes revealed less ocular irritation among iDose users, presumably thanks to reduced reliance on drops.
Some of these pivotal trials followed patients beyond 12 months, noting that iDose continued to effectively release medication over extended periods. If needed, the device could be replaced or refilled in a follow-up procedure. Data from these research efforts have been submitted to regulatory agencies like the U.S. Food and Drug Administration (FDA), paving the way for market approval and wider clinical adoption.
Comparative Efficacy and Safety
While direct comparisons between iDose and other micro-invasive treatments—such as trabecular bypass stents—are still limited, a few studies have begun exploring how the device pairs with MIGS procedures or complements conventional surgeries. Notably:
- Combined Procedures: Some clinicians place the iDose simultaneously with cataract surgery or MIGS implants. Preliminary data suggest that combining efforts to lower IOP through mechanical outflow enhancement with the chemical control of iDose can yield synergistic benefits.
- Reduced Topical Medication Load: Participants who received iDose typically scaled back or discontinued many, if not all, of their topical regimens. This not only eased daily routines but also minimized side effects like hyperemia or dryness often associated with eye drops.
It’s important to note that not all patients have identical responses. Factors such as baseline IOP, severity of glaucoma, ocular anatomy, and individual healing patterns can shape outcomes. Still, the consensus from academic publications consistently emphasizes that iDose is both safe and effective, particularly for those challenged by daily medication adherence.
Ongoing Research and Future Directions
Scientific and clinical interest in sustained-release glaucoma therapies continues to grow. Several lines of investigation could further refine and expand iDose use cases:
- Refillable Systems: Next-generation iDose devices may be designed for quick and simple refills, eliminating the need to remove the implant altogether.
- Alternative Drug Formulations: While prostaglandin analogs remain the mainstay for many patients, there is scope for iDose variants loaded with different medication classes, like beta-blockers or carbonic anhydrase inhibitors, for those who respond best to alternate therapies or are intolerant of certain agents.
- Longer Follow-Up Studies: Five-year or even 10-year data would offer invaluable insight into how iDose performs as a truly long-term solution, particularly in patients who receive it at earlier stages of glaucoma.
- Pediatric and Complex Cases: While adult-onset open-angle glaucoma is the primary target, some specialists wonder if sustained-release implants might help manage congenital or secondary forms of glaucoma in more complicated scenarios.
Thus far, the results point to iDose as a game-changer. By demonstrating consistent IOP control, the device addresses a critical gap in glaucoma care: bridging the distance between effective medications and real-world adherence. For patients at risk of vision loss due to inconsistent therapy, iDose may soon become a standard tool in the modern glaucoma arsenal.
Proven Impact on Pressure Control and Vision Health
Glaucoma’s hallmark—an elevated intraocular pressure that gradually damages the optic nerve—demands vigilant control to preserve vision. Clinical experience and published research consistently show that sustained-release solutions like Glaukos iDose can stabilize pressure effectively, particularly in patients who struggle with daily drop regimens. Below, we delve deeper into the device’s specific impact on lowering IOP and outline its broader implications for vision maintenance and patient safety.
Reliable IOP Reduction Over Time
One of the fundamental selling points of iDose is its potential to maintain lower, more predictable IOP levels. Topical eye drops, while effective in theory, rely heavily on the patient’s routine compliance. Missed doses or improper application can lead to fluctuating drug concentrations within the eye, resulting in inconsistent IOP control. iDose bypasses this issue by using a controlled release mechanism that releases medication at a steady rate.
Many patients who transition from eye drops to iDose report fewer IOP spikes and dips. In clinical assessments:
- Early Post-Implant Stability: IOP often stabilizes within the first few weeks, once the ocular environment adjusts to the implant.
- Long-Term Trajectory: Published data and real-world observations suggest that stable, lowered pressures can persist for 12 to 24 months or more, dependent on the design iteration and the patient’s individual physiology.
- Lower Peak Pressures: Some individuals see a notable reduction in their highest daily IOP levels (peak pressures), reducing the risk of optic nerve compromise.
Preserving the Optic Nerve and Visual Fields
While controlling IOP is a quantifiable metric, the real success of any glaucoma therapy ultimately lies in preserving the optic nerve and preventing further vision loss. Evaluations such as visual field testing (often using automated perimetry) provide a window into how well iDose users are safeguarding their peripheral vision. Patients using iDose alongside regular follow-ups often display slower progression of visual field defects compared to counterparts relying solely on self-administered drops.
Regular OCT imaging (optical coherence tomography) can also reveal how stable the retinal nerve fiber layer remains once iDose is in place. Reduced thinning of this layer over time indicates that the optic nerve is receiving better protection from harmful IOP spikes.
Patient Comfort and Adherence Gains
A secondary but crucial element of vision protection hinges on adherence. By eliminating the need to remember multiple daily drops, iDose addresses the most common pitfall of medication-based glaucoma management. Some patients are simply more likely to skip or forget drops, especially if their condition is asymptomatic or if the eye drops cause side effects like stinging or redness.
When combined with routine follow-up, iDose ensures:
- Consistency: No daily routine to remember, beyond normal eye care check-ups.
- Fewer Surface Side Effects: Because the drug is delivered internally, many patients experience fewer episodes of dryness, irritation, or eyelid inflammation.
- Better Quality of Life: Without constant reminders to apply drops, many people report enhanced peace of mind, which can improve overall acceptance of their glaucoma diagnosis.
Of course, some patients who previously used multiple classes of eye drops may still need additional therapies—particularly if they have advanced or complex glaucoma. Nonetheless, iDose often reduces the total burden, which can still lead to significant improvements in overall compliance.
Balanced Risk Profile
All surgical or device-based glaucoma interventions carry a degree of risk. However, iDose’s minimally invasive insertion technique and secure anchoring significantly limit complications. Over the course of follow-ups, ophthalmologists track endothelial cell counts to ensure that the device does not contact the cornea excessively, which could lead to cell loss. Thus far, published data suggest that corneal health remains robust among iDose recipients.
Potential complications include:
- Mild Postoperative Inflammation: Typically managed with a short course of steroid eye drops.
- Transient Rise in IOP: In rare cases, the immediate postoperative period can witness a spike in eye pressure, although this often resolves once the implant begins releasing medication and ocular fluid dynamics balance out.
- Device Malposition: Very rare but can occur if the implant shifts. Surgeons minimize this risk by ensuring correct placement and verifying device stability before concluding the procedure.
Compared to more invasive glaucoma surgeries (like trabeculectomy), iDose generally involves a shorter recovery and less stringent postoperative management. This is especially appealing for older adults or those with multiple comorbidities who need to avoid lengthy hospital stays or complex wound care.
Given these findings, iDose appears to deliver significant IOP-lowering effects with fewer of the pitfalls that plague traditional drop regimens. By combining reduced day-to-day hassle with consistent medication release, the implant empowers patients to protect their vision long term, potentially averting disease progression and the associated challenges of advanced glaucoma.
Cost Options for iDose and Coverage Details
Costs for the iDose procedure vary, influenced by factors such as clinic fees, surgeon expertise, and geographical region. Some eye centers charge a bundled price that includes the implant, the surgical insertion, and short-term postoperative care. These fees may range from a few thousand dollars to more substantial amounts, depending on the intricacy of the procedure and local market rates. Insurance coverage typically depends on the patient’s plan, with some private insurers and government programs offering partial or full reimbursement when iDose is deemed medically necessary. Financing or payment plans are often available at specialized eye clinics, giving patients an accessible path to this novel treatment option.
Disclaimer: This article serves an educational purpose and should not replace personalized medical advice. Always consult your ophthalmologist or healthcare provider for guidance tailored to your individual situation.
We invite you to share this article with others via Facebook, X (formerly Twitter), or any other social media platforms you prefer. Spreading the word helps more people learn about cutting-edge treatments like iDose®, potentially safeguarding their sight for years to come.