Retinal degenerative conditions such as retinitis pigmentosa and certain forms of advanced macular disease can lead to progressive vision loss, often culminating in near or total blindness. For decades, the medical community’s mainstay approaches to managing these disorders included low-vision aids, mobility training, and limited pharmacological options aimed at slowing disease progression. However, these measures offered few prospects for regaining meaningful visual perception. In response, researchers and clinicians turned to high-technology solutions, exploring ways to bypass damaged photoreceptors altogether by stimulating the retina’s remaining neural cells.
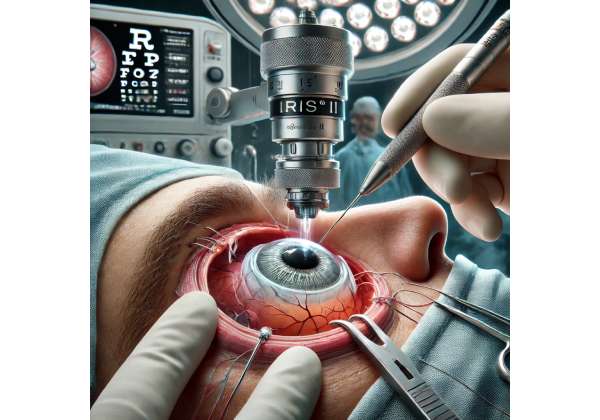
Among the most innovative breakthroughs in this domain is the IRIS® II epiretinal implant. Designed to restore basic visual function in individuals with profound retinal damage, this cutting-edge prosthetic device leverages advanced microelectronics, miniaturized cameras, and sophisticated algorithms to convert light into electrical signals interpretable by the brain. While not a panacea or a means of regaining fully normal sight, the IRIS® II system provides an unprecedented level of assistance to those with degenerative retinal conditions, allowing them to detect shapes, movement, and contrasts that can significantly improve daily navigation and quality of life. By combining surgical precision, state-of-the-art bioengineering, and patient-centric rehabilitation, IRIS® II stands at the forefront of efforts to help patients reclaim some degree of visual independence.
Understanding the Fundamental Features of IRIS® II
Development of epiretinal implants marks a significant milestone in biomedical technology. As the retina degenerates, the link between photoreceptors and neural pathways weakens. Yet, the upstream retinal ganglion cells often remain at least partly functional, and their axons form the optic nerve carrying visual information to the brain. The IRIS® II prosthesis capitalizes on this anatomical reality by delivering controlled electrical stimuli directly to retinal ganglion cells or inner retinal layers, bypassing the damaged photoreceptors.
Key Components and How They Interact
A typical IRIS® II system consists of several interdependent parts, each engineered to handle a specific aspect of capturing, processing, and relaying visual information:
- External Vision Capture:
The user wears a pair of specialized glasses equipped with a mini-camera. This high-resolution camera captures the external visual scene in real time. Recorded images pass through an external processor that converts them into simplified signals suitable for neural stimulation. - External Processing Unit:
Often small enough to be worn on a belt or in a pocket, this processing unit breaks down visual input into patterns of contrast, edges, and light intensity. The ultimate goal is to produce a stimulus array corresponding to salient features of the environment, such as doorways or obstacles. - Implantable Retinal Device:
The heart of IRIS® II is the epiretinal microelectrode array, placed surgically on the surface of the retina (the epiretinal region). Tiny electrodes—measured in micrometers—deliver electrical pulses to the retinal cells. The number and arrangement of electrodes directly influence the resolution and clarity of perceived images. - Wireless Power and Data Transmission:
A coil or inductive link ensures that minimal hardware resides within the body. By wirelessly sending power and data signals from the external module to the implanted device, the system avoids bulky batteries or connectors that could introduce infection risks or mechanical issues.
Why Epiretinal Placement Matters
Retinal prostheses can be placed in different locations, including subretinal (beneath the retina) or epiretinal (on top of the retina). The IRIS® II design advocates for an epiretinal approach for several reasons:
- Direct Access to Retinal Ganglion Cells: By resting on the inner surface, the electrodes are near the final output neurons leading to the optic nerve, enabling more direct control over the visual signals transmitted to the brain.
- Reduced Surgical Complexity: Certain subretinal implants require more invasive procedures to lift the retina. With epiretinal placement, surgeons typically perform a pars plana vitrectomy, then position the implant onto the retina with relative precision.
- Potential for Removability or Upgrades: Some epiretinal systems are designed in ways that facilitate easier removal if an upgrade or major revision becomes necessary in the future.
Confirmed Advantages Over Previous Generations
Earlier prototypes of retinal prosthetics were limited by small electrode counts, bulky external hardware, or suboptimal power transmission. IRIS® II addresses many of these limitations:
- Enhanced Electrode Array: The IRIS® II system typically offers more electrodes than older designs, providing finer-grained stimulation and better pattern recognition potential for the user.
- Bio-Inspired Pixel Processing: By simulating natural retina function—emphasizing contrast edges and movement detection—the IRIS® II aims to deliver more interpretable signals to the user’s visual cortex.
- Modular Upgradability: Manufacturers of the IRIS® II implant have considered future expansions. In some versions, software updates may improve image processing algorithms without necessitating further invasive interventions.
With these advancements, the IRIS® II epiretinal implant is positioned as a promising solution for patients with advanced degenerative disease, especially those for whom alternative therapies have exhausted their potential. Nevertheless, achieving consistent success depends on more than just the hardware: surgical technique, postoperative training, and patient commitment all shape the final visual outcomes. In the next section, we delve into the protocols guiding IRIS® II implementation from initial consultation to follow-up care.
Steps to Implantation and Long-Term Use
Successfully leveraging IRIS® II for vision restoration requires a methodical approach encompassing patient screening, careful surgery, and dedicated rehabilitation efforts. While the technology itself is advanced, real-world clinical benefits rely on precise coordination across multiple medical specialties—ophthalmology, neuro-ophthalmology, rehabilitation, and sometimes psychology.
Assessing Suitability for IRIS® II
Not all patients with retinal degeneration are prime candidates for an epiretinal implant. Medical teams must consider multiple factors to maximize the device’s potential:
- Confirming Advanced Degeneration: Typically, the IRIS® II is recommended for individuals with severe retinal disease (like retinitis pigmentosa) who have minimal or no remaining functional vision. Those with some preserved vision might find conventional aids more appropriate.
- Viability of the Optic Nerve: The device depends on intact or partially functioning ganglion cells and an optic nerve capable of relaying signals. If the optic nerve is irreversibly damaged (due to long-term glaucoma, for instance), the implant’s utility diminishes.
- General Health and Surgical Tolerance: Because IRIS® II implantation requires a vitrectomy and electrode array placement, candidates must be in suitable health for eye surgery, with stable blood pressure and no unmanageable bleeding disorders.
- Patient Motivation and Support System: Postoperative training for retinal implants is extensive. Patients must commit to device calibration sessions, visual training, and ongoing follow-up to achieve meaningful outcomes. A supportive caregiver or family environment often strengthens the likelihood of success.
Surgical Procedure and Hospital Stay
Once a patient is deemed eligible, the surgical team arranges the implantation date. Here’s a step-by-step outline:
- Preoperative Consultation: The ophthalmologist discusses the procedure’s details, reviewing potential risks like infection, retinal detachment, or device malfunction. The patient is given instructions about fasting or discontinuing certain medications if necessary.
- Anesthesia and Vitrectomy: On the day of surgery, local or general anesthesia is administered. A pars plana vitrectomy is performed to remove the vitreous humor, granting the surgeon clear access to the retina.
- Placement of the Epiretinal Array: With microsurgical instruments, the electrode array is gently placed onto the retina’s surface. In some cases, small tacks or adhesives secure it to prevent shifting. Surgeons must ensure that the array lines up with critical regions of the macula or surrounding retina to optimize signal generation.
- Connection to External Components: A thin lead or cable might be tunneled to a receiving coil or electronics module, typically placed under the skin near the temple. This step enables wireless data transfer and power supply from the external camera and processing unit.
- Closure and Recovery: The incisions are sealed, and an eye patch or protective shield is applied. Patients often remain under observation for one or two nights, depending on the center’s protocols and any immediate postoperative complications.
Early Postoperative Phase
During the initial recovery period, the implanted eye may feel sore or irritated. Physicians prescribe antibiotic drops to prevent infection, alongside anti-inflammatory medication to manage swelling. Regular check-ups in the first weeks ensure that:
- The retina remains attached and that fluid or hemorrhages are absent.
- The incision sites heal normally without signs of infection or inflammation.
- The device registers proper signals when tested with external hardware. Surgeries typically involve verifying the electrical connections by gently activating some electrodes to confirm neural response.
Calibration, Rehabilitation, and Ongoing Support
Achieving functional use of IRIS® II extends well beyond a successful operation. Extensive training helps patients interpret the newly generated visual patterns:
- System Activation
Typically, the device is switched on a few weeks after surgery to allow initial healing. A specialized technician or low-vision therapist calibrates the external camera’s parameters, adjusting brightness, contrast, and electrode stimulation patterns to suit the patient’s threshold levels. - Visual Training
With guidance from occupational therapists or vision rehabilitation specialists, the patient learns how to interpret the device’s signals. Activities might include locating bright objects on a contrasting table, recognizing basic shapes, or detecting door frames in an actual environment. - Incremental Complexity
Over time, therapy sessions become more challenging—introducing tasks such as object sorting or following moving targets. By systematically increasing complexity, patients gradually refine their ability to exploit the device’s stimuli for real-world tasks like navigation and item identification. - Lifelong Maintenance and Upgrades
Routine follow-ups verify that the implant remains stable. If technology advances, the external hardware or software may be upgraded to improve resolution or introduce new features (like dynamic edge detection). Rarely, if a hardware malfunction arises, revision surgery might be necessary to replace faulty components or reposition the electrode array.
The journey from candidacy evaluation to functional use typically spans several months. However, many recipients highlight that the regained ability—even if modest compared to normal vision—transforms their day-to-day experience, fostering greater independence and connectivity to their surroundings. Whether it’s navigating a familiar corridor or discerning a loved one’s silhouette, the IRIS® II can profoundly change how someone with advanced retinal disease engages with the world.
Reviewing Clinical Evidence and Real-World Outcomes
Technological breakthroughs in bionic vision systems are shaped largely by accumulated clinical data. Since its introduction, the IRIS® II implant has undergone rigorous evaluation in controlled studies and observational cohorts, enabling researchers and surgeons to refine surgical protocols, identify the best candidate profiles, and strengthen device reliability. Below is an overview of relevant research findings and ongoing investigations.
Highlights from Clinical Trials
Before widespread clinical use, IRIS® II was tested in carefully designed trials measuring both safety and the level of functional vision restoration:
- Feasibility and Early Safety: In initial feasibility studies, small groups of patients with end-stage retinal degeneration were implanted. Monitored over months, outcomes demonstrated stable fixation of the array with no severe adverse events like persistent hypotony (extremely low intraocular pressure) or repeated retinal detachments.
- Functional Outcomes: A subset of patients reported improved light perception and the ability to detect motion or large shapes. While the number of electrodes in an epiretinal implant cannot replicate the resolution of a healthy retina, many participants found that even rudimentary signals greatly assisted with orientation and mobility.
- Reliability Data: Longer-term follow-ups highlight device retention, with a small percentage requiring re-intervention. Instances of partial electrode failure occurred but were often addressed via external reprogramming or relatively minor surgical adjustments.
Observational Reports and Patient Testimonials
Beyond formal trials, doctors and implant recipients share anecdotal accounts of the technology’s everyday impact:
- Navigation Gains
Patients frequently mention the device’s help in navigating around furniture, identifying doorways, and spotting high-contrast objects such as white plates on a dark table. This practical advantage reduces collisions and fosters greater confidence in unfamiliar settings. - Increased Social Interaction
Some individuals describe how the IRIS® II system allows them to detect gestures or broad facial expressions, improving their social engagement. Although recognizing fine details like reading expressions is often not feasible, even basic outlines of companions can enhance communication. - Rehabilitation Success
The learning curve for translating epiretinal signals into meaningful “sight” is substantial. Patients who diligently attend rehabilitation sessions show more pronounced improvements, underscoring the need for comprehensive support that includes orientation-and-mobility training plus psychosocial counseling.
Emergent Avenues for Further Research
Ongoing research seeks to increase the IRIS® II’s electrode density, refine external imaging algorithms, and explore synergy with other therapies:
- Combined Approaches
In advanced degenerations, complementary therapies like retinal cell transplant or gene therapy could preserve existing retinal structures while the epiretinal device supplies artificial input. Such integrated strategies may optimize residual vision while bridging gaps in lost photoreceptor function. - Neuroplasticity Studies
By examining how the brain’s visual cortex adapts to electrically generated patterns, neuroscientists hope to identify ways to accelerate or deepen the device’s integration. Some investigations look at harnessing transcranial magnetic stimulation or other modalities to boost cortical plasticity, potentially leading to sharper image perception. - Long-Term Durability
The microelectronics used in the IRIS® II are subject to mechanical and electrical stress within the eye’s fluid environment. Researchers continue evaluating novel materials, hermetic seals, and robust coatings that extend device longevity with minimal mechanical complications.
Taken as a whole, the available evidence underscores that the IRIS® II fosters real, tangible benefits for many patients with severe retinal degeneration. While it cannot replicate the crisp detail of natural sight, it does open doors to new forms of independence, environmental awareness, and community engagement. The entire field of bionic vision stands at an exciting crossroads, and the IRIS® II remains a prime illustration of how technology can seamlessly blend with biology to overcome previously insurmountable sensory deficits.
Evaluating Impact and Ensuring Well-Being
When it comes to assessing whether the IRIS® II epiretinal implant is truly effective and safe, multiple metrics come into play. Performance is not solely measured by medical endpoints like device survival or electrode function; it also involves gauging patient satisfaction, psychological changes, and improvements in daily living. By understanding these dimensions, clinicians and prospective users can make informed decisions about the therapy’s suitability.
Functional Achievements and Quality-of-Life Indicators
Many individuals living with advanced retinal disease learn orientation and mobility skills that do not rely heavily on vision. However, the capacity to distinguish shapes or movement can streamline these tasks. Common ways to gauge the IRIS® II’s value include:
- Light and Motion Detection
Before the surgery, patients might only perceive vague flashes or none at all. Post-implant, detecting direction of motion—say, a person walking across the room—offers a substantial leap in spatial understanding. - Object Localization
Activities like locating doors or windows in a new room become easier as the user perceives brightness differentials. Even tasks such as placing an object within a specified area (e.g., a tray or table corner) become more accurate. - Self-Reliance
Some patients regain the confidence to navigate short distances without constant assistance. Although a cane or guide dog might still be necessary, the implant’s cues reduce the risk of bumping into large obstacles. - Psychosocial Benefits
Renewed engagement with surroundings often translates to better emotional well-being. Reports of decreased isolation and improved mood reflect a sense of regained control, even if the visual details remain limited.
Risk-Benefit Profile
As with any invasive device, the IRIS® II entails certain risks:
- Possible Complications
- Infection: Though uncommon, ocular infections can arise from the surgery or subsequent hardware-related issues.
- Device Failure: Electrical malfunctions or broken leads could demand reoperation. Advances in robust design have lowered these rates, but they persist as a real albeit low-probability scenario.
- Retinal Damage: Excess current intensity or mechanical pressure from the electrode array might harm adjacent retinal tissue. Careful calibration and stable device positioning help mitigate this concern.
- Mitigating Factors
- Regular Follow-Up: Consistent monitoring for early signs of mechanical wear or infection can prevent more severe complications.
- Individualized Stimulation Parameters: Surgeons and device specialists tailor pulse intensities to each patient’s threshold, lowering the odds of overstimulation.
- Advanced Surgical Techniques: With refined instrumentation and improved visualization technology, vitreoretinal surgeons can place the array more confidently, reducing trauma to the eye.
- Comparative Safety
Compared to older-generation implants or other invasive procedures (like a complete retina transplant, which is not clinically standard yet), the IRIS® II is considered relatively safe. Serious complications are infrequent, and many can be managed effectively if caught early.
Making Sense of Variable Outcomes
Not all IRIS® II recipients attain identical improvements. Variations may stem from:
- Disease Severity: Those who still have some functioning inner retinal layers often adapt more swiftly, benefiting from synergy between residual natural vision and the implant’s signals.
- Neuroplasticity: The brain’s ability to interpret artificial stimuli can vary widely. Younger patients or those with shorter durations of blindness sometimes exhibit a faster learning curve.
- Rehabilitation Commitment: Time spent on structured visual rehabilitation correlates strongly with better outcomes. Patients who commit to practice and training are more likely to realize the device’s full potential.
Nonetheless, the overall data consistently affirm that IRIS® II can afford significant advantages to a select group of individuals for whom other treatments have failed to preserve or restore functional sight. This makes the epiretinal implant a valuable resource in the continuum of care for severe retinal degenerations, filling a critical gap between routine low-vision rehabilitation and purely experimental regenerative interventions.
Cost Considerations and Potential Financing Options
The IRIS® II epiretinal implant, as an advanced medical technology, involves considerable expense for both the device itself and the associated surgical and rehabilitation services. Although costs can differ by country, medical facility, and insurance coverage, a typical total investment can range between \$90,000 and \$150,000 per implant. This figure commonly covers preoperative assessments, the surgical procedure, the implant hardware, and initial follow-up visits. Some clinics offer financing plans or direct patients to charities and grants that might offset the expense. Insurance reimbursement varies, but limited coverage may be available depending on the policy, diagnosis, and local healthcare regulations. Prospective patients are advised to request comprehensive cost breakdowns and explore all available funding sources before committing to the procedure.
Disclaimer:
This article is provided for informational purposes only and does not replace professional medical advice. Always consult a qualified healthcare provider for personalized guidance on any therapy or procedure mentioned here.
We invite you to share this article with friends, family, and anyone who might be exploring bionic vision solutions like the IRIS® II epiretinal implant. Use our Facebook and X (formerly Twitter) share buttons or your preferred social media platforms to spread the word and help others learn about new possibilities in restoring functional vision.