Primary Congenital Glaucoma (PCG) is a rare but severe type of glaucoma that develops early in life, usually within the first few months or years after birth. This condition is defined by an abnormal development of the eye’s drainage system, which causes increased intraocular pressure (IOP), resulting in optic nerve damage and potential vision loss. Unlike other types of glaucoma that develop later in life, PCG is present from birth, though symptoms may not be immediately apparent.
Anatomy and Physiology
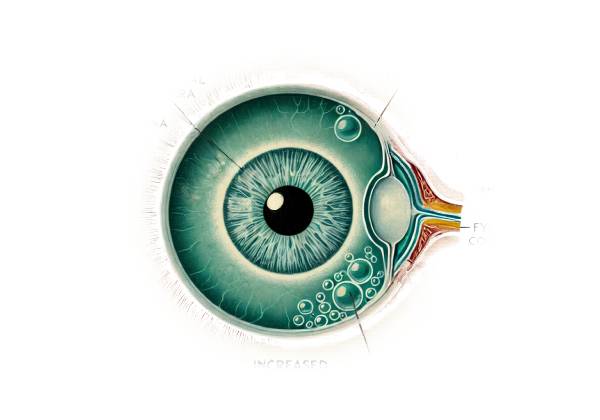
Understanding Primary Congenital Glaucoma requires a thorough understanding of the eye’s basic anatomy and physiology, particularly the structures involved in fluid regulation and drainage. The eye produces aqueous humor, a clear fluid that nourishes and shapes the eye. The ciliary body produces aqueous humor, which flows through the pupil before draining out of the eye via the trabecular meshwork and Schlemm’s canal, which are located at the angle where the iris meets the cornea.
PCG has malformed trabecular meshwork and Schlemm’s canal, resulting in poor aqueous humor drainage. This poor drainage causes elevated IOP, which can harm the optic nerve, the vital structure that transmits visual information from the eye to the brain.
Causes and Risk Factors
Genetic mutations that affect the development of the eye’s drainage system are the primary cause of primary congenital glaucoma. The condition is autosomal recessive, which means that a child must inherit two copies of the defective gene, one from each parent, in order to develop it. CYP1B1, LTBP2, and TEK are some of the known genes associated with PCG. However, not every case has a known genetic cause, and other factors may also play a role in PCG development.
Symptoms
PCG symptoms can range in severity, but the condition frequently presents with a distinct set of signs that can alert caregivers and healthcare providers to its presence. These symptoms usually include:
- Enlarged Eyes (Buphthalmos): The increased IOP can cause the eye to enlarge, resulting in a condition known as buphthalmos. This is especially noticeable in infants and young children, whose eye tissues are more elastic and prone to stretching.
- Corneal Clouding: High IOP can make the cornea cloudy or hazy, impairing vision.
- Tearing (Epiphora): Excessive tearing is common and may be associated with frequent eye rubbing.
- Photophobia: Light sensitivity is a common complaint as a result of corneal changes and elevated IOP.
- Blepharospasm: An involuntary tightening of the eyelids in response to pain or light sensitivity.
Epidemiology
Primary congenital glaucoma is a rare condition that affects approximately 1 in 10,000 to 1 in 20,000 live births worldwide. The prevalence varies by geographic region and ethnic background, with higher rates reported in some populations, such as those of Middle Eastern descent. Males are slightly more commonly affected than females, with a male-to-female ratio of about 3:2.
Pathophysiology
The pathophysiology of PCG consists of several key abnormalities in the development of the anterior segment of the eye:
- Trabeculodysgenesis: Trabeculodysgenesis is the primary defect in PCG, characterized by abnormal development of the trabecular meshwork and Schlemm’s canal. This causes impaired aqueous humor drainage and elevated IOP.
- Genetic Mutations: Mutations in genes such as CYP1B1 interfere with the normal development and function of the trabecular meshwork. These genetic mutations can cause structural abnormalities that prevent the proper flow of aqueous humor.
- Structural Changes: Elevated IOP can cause structural changes in the eye, such as corneal enlargement, edema, and optic nerve cupping. If not treated promptly, these changes can cause permanent vision impairment.
Prognosis
The prognosis for children with primary congenital glaucoma is determined by their age at diagnosis and the effectiveness of treatment. Early detection and timely surgical intervention significantly increase the chances of preserving vision. Without treatment, PCG can cause severe vision impairment or blindness due to progressive optic nerve damage.
Effects on Quality of Life
PCG has a significant impact on a child’s quality of life and development. Visual impairment can have an impact on learning, social interactions, and overall development. Furthermore, the need for frequent medical appointments and possible surgeries can be difficult for both the child and their family. Providing appropriate support and resources is critical to managing the condition and promoting the child’s well-being.
Complications
If left untreated, Primary Congenital Glaucoma can cause a number of serious complications:
- Permanent Vision Loss: Progressive optic nerve damage can lead to irreversible vision loss or blindness.
- Strabismus: Eye misalignment can impair vision and depth perception.
- Amblyopia: Also known as “lazy eye,” this condition can develop as a result of insufficient visual input from the eyes.
- Corneal Scarring: Persistently elevated IOP can cause corneal scarring, which impairs vision.
- Psychosocial Effects: The chronic nature of the condition and its impact on vision can cause psychological and social difficulties for the child and their family.
Research and Advances
The ongoing research aims to improve our understanding and management of PCG. Advances in genetic testing and molecular biology are assisting in identifying the underlying genetic causes of the condition, which could lead to targeted therapies in the future. Furthermore, advances in surgical techniques and early detection methods continue to improve the outcomes for affected children.
Diagnostic Techniques for Primary Congenital Glaucoma
Primary Congenital Glaucoma requires a multifaceted approach that includes clinical evaluation, specialized tests, and imaging studies. Early and accurate diagnosis is critical for initiating treatment and avoiding irreversible vision loss.
Clinical Evaluation
- Comprehensive Eye Examination: A thorough examination by a pediatric ophthalmologist is the foundation for diagnosing PCG. This includes evaluating the corneal diameter, intraocular pressure (IOP), and optic nerve function. To ensure an accurate assessment, young children are often sedated or anesthetized during the examination.
- Intraocular Pressure Measurement: An elevated IOP is an important diagnostic indicator of PCG. Tonometers used to measure IOP include the Goldmann applanation tonometer, rebound tonometer, and Perkins tonometer. To ensure accurate readings, infants and young children may require sedation during measurement.
- Gonioscopy: This procedure uses a specialized lens to visualize the anterior chamber angle structures. It aids in identifying abnormalities in the trabecular meshwork and Schlemm’s canal that may indicate PCG. Gonioscopy provides important information about the anatomy of the angle as well as the presence of any developmental anomalies.
Imaging Studies
Imaging studies are useful tools for diagnosing PCG and determining the extent of ocular damage:
- Ultrasound Biomicroscopy (UBM): UBM generates high-resolution images of the anterior segment structures, such as the trabecular meshwork, ciliary body, and anterior chamber angle. It aids in the identification of structural abnormalities as well as the assessment of the developmental defect’s severity.
- Optical Coherence Tomography (OCT): OCT is a non-invasive imaging technique for obtaining detailed cross-sectional images of the retina and optic nerve. It is useful for detecting optic nerve head changes, such as cupping, which are indicative of glaucoma.
- Corneal Topography: This imaging technique maps the surface curvature of the cornea, which aids in detecting corneal enlargement and irregularities associated with PCG.
Additional Diagnostic Tests
Additional tests may be required to confirm the diagnosis and assess the severity of the condition.
- Corneal Diameter Measurement: Determining the corneal diameter is critical for diagnosing PCG. An enlarged corneal diameter (greater than 11 mm in newborns and greater than 12 mm in infants) is an important diagnostic clue.
- Optic Nerve Assessment: A thorough examination of the optic nerve for signs of cupping, pallor, and other abnormalities is critical in determining the extent of optic nerve damage. This assessment is typically performed with ophthalmoscopy, which allows the pediatric ophthalmologist to see the optic nerve head directly.
Genetic Testing
When a genetic cause is suspected, genetic testing can be used to identify mutations linked to Primary Congenital Glaucoma. Mutations in genes such as CYP1B1, LTBP2, and TEK can confirm a diagnosis and provide useful information for genetic counseling.
Differential Diagnosis
Accurate diagnosis of PCG necessitates distinguishing it from other conditions that can cause similar symptoms. Differential diagnosis includes:
- Juvenile Open-Angle Glaucoma: This type of glaucoma affects older children and adolescents and is characterized by the absence of buphthalmos and corneal enlargement seen in PCG.
- Secondary Congenital Glaucoma: This condition occurs in conjunction with other ocular or systemic abnormalities, such as Axenfeld-Rieger syndrome, Sturge-Weber syndrome, or neurofibromatosis.
- Corneal Disorders: Conditions such as congenital hereditary endothelial dystrophy or sclerocornea can cause corneal clouding and enlargement, resembling PCG.
- Infantile Glaucoma: Similar to PCG, but may develop later in infancy or early childhood.
Primary Congenital Glaucoma Management
Managing Primary Congenital Glaucoma (PCG) necessitates a multidisciplinary approach that includes medical therapy, surgery, and ongoing monitoring. The primary goal of treatment is to lower intraocular pressure (IOP) to avoid optic nerve damage and preserve vision. Here are the main methods for managing PCG:
Medical Management
- Topical Medications: While medical therapy is frequently insufficient as a standalone treatment for PCG, it can be used to stabilize IOP prior to surgery or as an adjunctive therapy afterward. Commonly used medications include:
- Beta-blockers: Medications such as timolol reduce aqueous humor production, lowering IOP.
- Carbonic Anhydrase Inhibitors: Drugs like dorzolamide and brinzolamide reduce aqueous humor production.
- Alpha Agonists: Brimonidine lowers IOP by decreasing aqueous humor production while increasing uveoscleral outflow.
- Prostaglandin Analogues: These drugs, such as latanoprost and bimatoprost, stimulate aqueous outflow but are less commonly used in infants due to potential side effects.
- Systemic Medications: If topical medications are ineffective, oral carbonic anhydrase inhibitors such as acetazolamide may be prescribed to further reduce IOP.
Surgical Management
Surgical intervention is the primary treatment for Primary Congenital Glaucoma. The choice of surgical procedure depends on the severity of the condition and the anatomical features of the eye.
- Goniotomy: This procedure involves making an opening in the trabecular meshwork to increase aqueous humor outflow. Goniotomy is usually performed with a specialized goniotomy knife or laser. It is most effective when the cornea is transparent enough to see the angle structures.
- Trabeculotomy: Like goniotomy, trabeculotomy entails opening the trabecular meshwork to improve aqueous outflow. This procedure can be done externally, making it appropriate for eyes with corneal clouding that prevents visibility of the angle structures.
- Combined Trabeculotomy-Trabeculectomy: This approach combines trabeculotomy and trabeculectomy to increase the chances of success. Trabeculectomy opens up an additional drainage pathway by forming a fistula between the anterior chamber and the subconjunctival space, allowing aqueous humor to bypass the obstructed trabecular meshwork.
- Drainage Devices: When traditional surgeries are unsuccessful or impractical, glaucoma drainage devices (tube shunts) can be implanted to aid in aqueous humor drainage. Examples include the Ahmed valve, the Baerveldt implant, and the Molteno implant.
- Cyclophotocoagulation: This laser procedure targets the ciliary body and reduces aqueous humor production. It is usually reserved for refractory cases in which other surgical options have failed.
Post-operative Care and Monitoring
Postoperative care is critical for ensuring the success of surgical interventions and avoiding complications:
- Medications: Anti-inflammatory and antibiotic eye drops are prescribed following surgery to prevent infection and inflammation. Adherence to the medication regimen is critical for successful healing.
- Follow-Up Visits: Regular follow-up visits are required to monitor IOP, evaluate surgical success, and detect complications early. The number of follow-up visits may decrease over time as the eye stabilizes.
- Additional Procedures: Some children may need multiple surgeries or other procedures to achieve adequate IOP control.
Supportive measures and rehabilitation
- Vision Therapy: Children with PCG may benefit from vision therapy and rehabilitation services to improve their visual function and development.
- Educational Support: By providing appropriate educational resources and support, children with visual impairments can succeed academically and socially.
- Parental Education and Support: Educating parents about the condition, treatment options, and the importance of sticking to follow-up appointments and medication regimens is critical for long-term success.
Trusted Resources and Support
Books
- “Pediatric Ophthalmology and Strabismus” by Kenneth W. Wright and Peter H. Spiegel
- This comprehensive book provides detailed information on various pediatric eye conditions, including Primary Congenital Glaucoma. It covers diagnostic methods, treatment options, and surgical techniques, making it a valuable resource for both students and practitioners.
- “Genetics for Ophthalmologists: The Molecular Genetic Basis of Ophthalmic Disorders” by Graeme C. M. Black
- This book explores the genetic aspects of ophthalmic disorders, including PCG. It provides insights into the genetic basis of the condition and discusses current and potential future genetic therapies.
Organizations
- American Academy of Ophthalmology (AAO)
- Website: www.aao.org
- The AAO offers extensive resources on various eye conditions, including Primary Congenital Glaucoma. Their website provides patient education materials, research updates, and professional guidelines to help patients and healthcare providers stay informed about the latest developments in ophthalmology.
- Glaucoma Research Foundation (GRF)
- Website: www.glaucoma.org
- The GRF provides valuable information on glaucoma types, treatment options, and ongoing research. They offer resources and support for patients and families affected by Primary Congenital Glaucoma, including educational materials and access to support networks.