What is traumatic glaucoma?
Traumatic glaucoma is a type of secondary glaucoma that develops following an eye injury. Traumatic glaucoma, as opposed to primary glaucoma, develops as a result of a specific ocular trauma. This condition is distinguished by an increase in intraocular pressure (IOP), which can cause damage to the optic nerve and progressive vision loss if not diagnosed and treated promptly. Traumatic glaucoma can develop immediately after an injury or months or even years later, necessitating long-term monitoring for people who have experienced eye trauma.
Etiology and Risk Factors
Traumatic glaucoma can result from a variety of eye injuries, such as blunt trauma, penetrating injuries, chemical burns, or radiation exposure. Each of these injuries can cause different pathological changes in the eye, contributing to increased intraocular pressure and the development of glaucoma.
Blunt Trauma
Blunt trauma, also known as closed-globe injury, is a leading cause of traumatic glaucoma. A blunt object, such as a fist, a ball, or a car airbag, can strike the eye and cause this type of injury. The force of the impact can cause several structural changes within the eye, which contribute to elevated IOP.
- Angle Recession: One of the most significant changes caused by blunt trauma is angle recession, which is defined as the tearing or separation of structures within the anterior chamber angle. The anterior chamber angle houses the trabecular meshwork, which drains aqueous humor from the eye. When the angle is recessed, it can disrupt the outflow of aqueous humor, resulting in increased IOP and, eventually, the development of glaucoma. Angle recession can be subtle and go unnoticed during the initial trauma, only to become noticeable years later as glaucoma progresses.
- Hyphema: The presence of blood in the anterior chamber of the eye is another common side effect of blunt trauma. The blood can obstruct the trabecular meshwork, preventing aqueous humor drainage and causing elevated IOP. While hyphema typically resolves on its own, there is still a risk of developing secondary glaucoma, especially if residual blood clots or inflammatory debris remain in the anterior chamber.
- Iridodialysis: Iridodialysis is the separation of the iris from its attachment to the ciliary body, which is often caused by blunt trauma. This condition can cause abnormal fluid dynamics within the eye, contributing to high IOP and the development of glaucoma.
- Lens Dislocation: Blunt trauma can cause the lens to dislocate from its original position. A dislocated lens can obstruct the flow of aqueous humor by physically blocking the trabecular meshwork or causing secondary inflammation, both of which can result in elevated IOP.
Penetrating Injuries
Penetrating injuries, also known as open-globe injuries, happen when a sharp object penetrates the eye and causes direct damage to its internal structures. These injuries are frequently associated with a high risk of infection, inflammation, and scarring, all of which can lead to the development of traumatic glaucoma.
- Scarring and Synechiae Formation: Penetrating injuries can cause scar tissue and synechiae, which are abnormal adhesions between the iris and other structures within the eye. These adhesions can obstruct the flow of aqueous humor, causing elevated IOP. Scarring in the trabecular meshwork can permanently impair function, resulting in chronic glaucoma.
- Intraocular Foreign Bodies: The presence of an intraocular foreign body (IOFB) after a penetrating injury can also lead to the development of glaucoma. The foreign body may cause direct damage to the trabecular meshwork or trigger a chronic inflammatory response, both of which can result in high IOP.
- Chemical and Thermal Burns: Chemical or thermal burns to the eye can severely damage the cornea, conjunctiva, and sclera, resulting in scarring and inflammation. These injuries can cause synechiae formation, trabecular meshwork dysfunction, and glaucoma.
- Sympathetic Ophthalmia: Sympathetic ophthalmia is a rare but serious complication of penetrating eye injuries, characterized by an autoimmune inflammatory response that can affect both injured and uninjured eyes. The resulting inflammation can severely damage the trabecular meshwork and other ocular structures, resulting in elevated IOP and the development of glaucoma.
Pathophysiology
The pathophysiology of traumatic glaucoma is complex and varies depending on the nature and severity of the initial injury. The common denominator in all cases of traumatic glaucoma is elevated IOP, which is caused by impaired aqueous humor outflow as a result of damage to the trabecular meshwork, anterior chamber angle, or other eye structures.
- Angle Recession Glaucoma: The tearing of the ciliary body following blunt trauma is a significant risk factor for the development of traumatic glaucoma. The ciliary body contains the trabecular meshwork, which drains aqueous humor from the eye. When the angle decreases, the trabecular meshwork may become dysfunctional, resulting in decreased outflow and elevated IOP. Angle recession is often asymptomatic and may go undetected until glaucoma develops years later.
- Inflammatory Glaucoma: Inflammation is a key factor in the development of traumatic glaucoma. Ocular trauma frequently activates an inflammatory response, resulting in the release of cytokines and other inflammatory mediators. These mediators can cause swelling and damage to the trabecular meshwork, reducing aqueous humor outflow. Chronic inflammation can also cause synechiae and scarring, exacerbating the condition.
- Ghost Cell Glaucoma: This rare type of secondary glaucoma can develop following trauma, especially if there has been a vitreous hemorrhage. Ghost cells are dead red blood cells that can migrate into the anterior chamber and obstruct the trabecular meshwork, causing elevated IOP. This type of glaucoma usually develops weeks or months after the initial trauma.
- Phacolytic Glaucoma: If the trauma damages the lens capsule, lens proteins may leak into the anterior chamber, causing an inflammatory response. Inflammatory cells and lens material can clog the trabecular meshwork, resulting in elevated IOP. Phacolytic glaucoma is more common in older people who have pre-existing cataracts because the lens proteins are more likely to leak from a weak lens capsule.
- Steroid-Induced Glaucoma: Corticosteroids are frequently used after ocular trauma to reduce inflammation. However, in some people, corticosteroid use can cause elevated IOP, resulting in steroid-induced glaucoma. This is more likely to happen in people who are predisposed to corticosteroid responsiveness, and it emphasizes the importance of closely monitoring IOP in patients undergoing steroid therapy following trauma.
Clinical Presentation
The clinical presentation of traumatic glaucoma varies according to the type and timing of the injury. In some cases, elevated IOP can be detected immediately after trauma, while in others, glaucoma develops gradually over time. The primary symptoms and signs of traumatic glaucoma are:
- Glaucoma is characterized by elevated intraocular pressure (IOP). Acute angle closure symptoms may include severe eye pain, headache, nausea, vomiting, and blurred vision in patients. However, in chronic cases, the increase in IOP may be asymptomatic and only detectable during routine eye exams.
- Vision Changes: Patients with traumatic glaucoma may experience gradual or sudden vision loss, depending on the severity and progression of the disease. Vision changes may include blurred vision, halos around lights, or decreased peripheral vision.
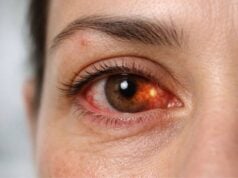
- Ocular Pain and Discomfort: In cases of acute angle closure or significant IOP elevation, patients may experience ocular pain, discomfort, or a sensation of pressure within the eye. Redness and photophobia (light sensitivity) are frequently associated with these symptoms.
- Hyphema: Blood in the anterior chamber (hyphema) is a common finding in blunt trauma and may be associated with elevated IOP. Hyphema may be visible to the naked eye or detected using a slit-lamp examination.
- Angle Recession: During gonioscopy, the anterior chamber angle may appear abnormally wide. Angle recession is often asymptomatic, but it can lead to chronic traumatic glaucoma.
- Corneal Edema: High IOP can cause corneal edema, which appears as a hazy or cloudy cornea. Patients may experience blurred vision or see halos around lights, especially in low-light situations.
- Irregular Pupil: Trauma can cause the pupil to become irregular or distorted, especially with iridodialysis or anterior chamber angle damage. An irregular pupil can be associated with abnormal light reflexes and vision changes.
Approaches to Diagnosing Traumatic Glaucoma
A comprehensive clinical evaluation, including a detailed patient history, thorough eye examination, and the use of specialized diagnostic tools, is required to diagnose traumatic glaucoma. The goal is to accurately measure intraocular pressure, identify any structural damage to the eye, and determine the root cause of the elevated IOP.
Clinical Examination
- Tonometry: Measuring intraocular pressure (IOP) is an important part of diagnosing glaucoma. Tonometry can be performed in a variety of ways, including Goldmann applanation tonometry, which is considered the gold standard. Elevated IOP is the primary indicator of glaucoma, and consistent readings above normal levels (10-21 mmHg) call for further investigation. In traumatic glaucoma, IOP may rise immediately following injury or gradually over time.
- Gonioscopy: Gonioscopy is required to evaluate the anterior chamber angle, which is where the trabecular meshwork is located. This examination allows the ophthalmologist to evaluate the angle’s structure and function, as well as detect abnormalities like angle recession, synechiae, or other damage that may impair aqueous humor outflow. In cases of traumatic glaucoma, gonioscopy can reveal angle recession, a subtle but significant finding that can predispose the patient to developing glaucoma.
- Slit-Lamp Examination: A slit-lamp examination allows for a detailed view of the eye’s anterior segment, which includes the cornea, iris, lens, and anterior chamber. This examination can detect signs of trauma, such as hyphema, iridodialysis, corneal edema, or lens dislocation. The presence of these symptoms, combined with elevated IOP, can strongly indicate traumatic glaucoma. The slit lamp can also help detect inflammatory cells in the anterior chamber, which may indicate ongoing inflammation and contribute to elevated IOP.
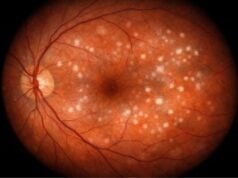
- Fundus Examination: A direct or indirect ophthalmoscope is used to thoroughly examine the optic nerve head (optic disc). The optic nerve head may exhibit glaucomatous damage, such as increased cupping or pallor. This examination is critical in determining the extent of optic nerve damage, which is an important indicator of glaucoma progression. In cases of traumatic glaucoma, the optic nerve may appear normal at first, but damage can occur over time if IOP remains elevated.
Imaging Studies
- Optical Coherence Tomography (OCT): OCT is a non-invasive imaging technique for obtaining high-resolution cross-sectional images of the retina and optic nerve head. It is used to measure the thickness of the retinal nerve fiber layer (RNFL) and the optic nerve head, both of which are susceptible to glaucoma damage. OCT can detect early structural changes in the optic nerve, preventing significant visual field loss. In traumatic glaucoma, OCT can help track the progression of optic nerve damage over time.
- Ultrasound Biomicroscopy (UBM): UBM is a sophisticated imaging technique that produces detailed images of the anterior segment structures, such as the ciliary body, iris, and anterior chamber angle. It is especially useful for measuring angle recession, detecting subtle changes in the trabecular meshwork, and determining the presence of foreign bodies or other trauma-related abnormalities. UBM can also be used to determine the position of a suspected lens dislocation.
- Visual Field Testing: Perimetry is an important diagnostic tool for determining the functional impact of glaucoma on a patient’s vision. This test assesses the patient’s peripheral vision and can detect glaucoma-related vision loss. Visual field testing is useful in monitoring the progression of traumatic glaucoma as well as the effectiveness of treatment.
- Anterior Segment Optical Coherence Tomography (AS-OCT): AS-OCT is a specialized type of OCT that examines the eye’s anterior segment. It allows for detailed visualization of the cornea, iris, and anterior chamber angle. AS-OCT can provide useful information about the angle’s structure as well as any trauma-related changes. It is especially useful when gonioscopy is inconclusive or when precise imaging of the angle structures is required.
- Fluorescein Angiography (FA): FA is used to assess retinal and choroidal circulation, particularly in cases where there is a suspicion of ischemic damage or choroidal detachment following trauma. Although not commonly used in the initial diagnosis of traumatic glaucoma, FA can be useful in determining the extent of retinal or choroidal involvement in complex cases.
Effective Approaches to Traumatic Glaucoma Management
Traumatic glaucoma management is complex and requires a tailored approach based on the type and severity of the injury, intraocular pressure (IOP), and extent of ocular structure damage. The primary goal is to reduce IOP to prevent further damage to the optic nerve and preserve vision. There are three types of management strategies: medical, surgical, and laser treatment.
Medical Management
Medical management is often the first line of treatment for traumatic glaucoma, especially in the early stages or when the IOP elevation is moderate.
- Topical medications:
- Beta-blockers: These medications, such as timolol, reduce the production of aqueous humor, lowering IOP. They are frequently used as the first-line treatment.
- Prostaglandin Analogues: Drugs such as latanoprost and bimatoprost increase the outflow of aqueous humor via the uveoscleral pathway, which helps to lower IOP. They are extremely effective and are frequently used once daily.
- Alpha Agonists: Brimonidine is an example of this class, which reduces aqueous humor production while increasing uveoscleral outflow.
- Carbonic Anhydrase Inhibitors: Dorzolamide and brinzolamide are common aqueous humor production reducers that are available in both topical and oral forms.
- Miotics: Pilocarpine may be used in certain cases to increase aqueous humor outflow by constricting the pupil, but it is less commonly used due to side effects.
- Oral medications:
- Oral Carbonic Anhydrase Inhibitors: Acetazolamide is an oral carbonic anhydrase inhibitor that can be used to quickly reduce IOP in acute situations. It is especially beneficial when topical treatments are insufficient.
- Hyperosmotic Agents: In an emergency, medications like mannitol can be used to quickly lower IOP by drawing fluid out of the eye and into the bloodstream. These are most commonly used in acute angle-closure glaucoma or other situations requiring a rapid reduction in IOP.
- Steroid therapy:
- Steroids are frequently prescribed to reduce inflammation, which can lead to increased IOP after trauma. However, their use must be closely monitored because they can sometimes worsen IOP elevation, leading to steroid-induced glaucoma.
Laser Treatment
When medical management alone is insufficient to control IOP, laser therapy is an option, as is addressing a specific anatomical abnormality with laser.
- Laser Trabeculoplasty:*
- This procedure uses laser energy to stimulate aqueous humor outflow through the trabecular meshwork. It is especially useful in cases where angle recession has resulted in trabecular dysfunction. Selective laser trabeculoplasty (SLT) is a popular technique that is minimally invasive and has a high safety profile.
- Laser Peripheral Iridotomy.
- When traumatic glaucoma is associated with angle-closure mechanisms, such as pupillary block or iris synechiae, laser peripheral iridotomy may be used. This procedure creates a small hole in the peripheral iris that allows aqueous humor to pass through, relieving pressure.
Surgical Management
Surgical intervention may be required in cases of traumatic glaucoma that are resistant to medical and laser treatments, or when significant structural damage needs to be repaired.
- Trabeculectomy:
- The most common surgical procedure for glaucoma is to create a new drainage pathway for aqueous humor by removing a portion of the trabecular meshwork. This surgery is effective at lowering IOP, but it has risks such as infection and hypotony (excessively low IOP).
- Glaucoma Drainage Devices :
- If trabeculectomy is not appropriate or has failed, glaucoma drainage devices (also known as aqueous shunts) may be implanted. These devices offer an alternative route for the drainage of aqueous humor from the eyes. The Ahmed valve and the Baerveldt tube are two commonly used devices.
- Cyclodestructive Procedures:
- Cyclophotocoagulation is a laser procedure that reduces aqueous humor production by focusing on the ciliary body, which produces the fluid. This procedure is frequently used as a last resort in cases of refractory glaucoma or when other surgical options are ineffective.
- Lenses Surgery:
- If lens dislocation or cataract formation contributes to high IOP, lens removal surgery may be necessary. This procedure can relieve the mechanical obstruction caused by the displaced lens and help restore normal aqueous humor dynamics.
- Goniosynechialysis:
- This is a surgical procedure that physically separates synechiae (adhesions) in the anterior chamber angle to allow aqueous humor outflow. It is especially useful for trauma-induced angle-closure glaucoma.
Post-operative Care and Follow-Up
Regardless of the treatment modality, close postoperative monitoring is essential to detect complications and keep IOP under control. To avoid disease progression, patients may need to monitor their IOP for the rest of their lives and make ongoing treatment adjustments. Regular visual field testing and optic nerve imaging are essential for assessing treatment efficacy and detecting signs of glaucoma progression.
Depending on the complexity of the case, traumatic glaucoma management frequently necessitates a multidisciplinary approach that includes collaboration between ophthalmologists, optometrists, and, in some cases, retinal surgeons or neuro-ophthalmologists.
Trusted Resources and Support
Books
- “Glaucoma: Clinical Care, Diagnosis, and Management” by John C. Morrison and Irvin P. Pollack: This book provides a comprehensive overview of the different types of glaucoma, including traumatic glaucoma, with a focus on diagnosis and management strategies.
- “Trauma and Emergency Care in Ophthalmology” by Urmi V. Shah and Pradeep V. Shah: This text offers detailed insights into the management of ocular trauma, including the various forms of secondary glaucoma that can result from such injuries.
Organizations
- American Academy of Ophthalmology (AAO): The AAO provides extensive resources on glaucoma, including guidelines for the diagnosis and management of traumatic glaucoma, and offers continuing education opportunities for ophthalmologists.
- Glaucoma Research Foundation (GRF): GRF is dedicated to advancing research and providing patient education on all forms of glaucoma, including traumatic glaucoma. The foundation offers a variety of resources for patients and healthcare providers alike.
- National Eye Institute (NEI): The NEI is a leading source of information on eye health, including research updates, clinical guidelines, and educational materials on traumatic glaucoma and other eye conditions.