Retinoblastoma is the most common malignant eye tumor affecting infants and young children. While technological advances have introduced various targeted approaches—ranging from chemotherapy to laser photocoagulation—cryotherapy remains a cornerstone for treating smaller retinal tumors. By using localized freezing to target cancerous growths, cryotherapy can preserve vision and effectively shrink tumors without resorting to more invasive interventions. Below is a comprehensive exploration of how cryotherapy is used in retinoblastoma management, covering everything from the condition’s genetic basis to treatment protocols and recent research findings.
1. A Closer Look at Cryotherapy for Retinoblastoma
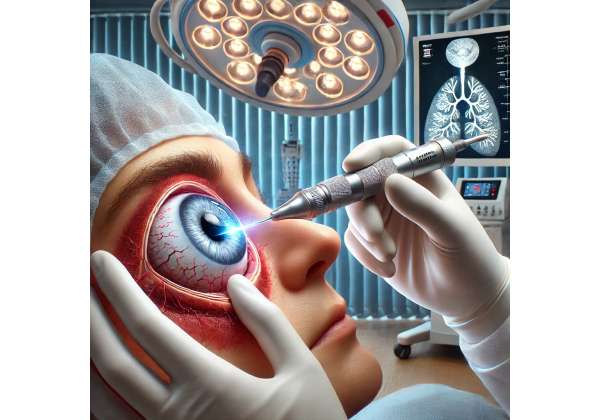
Cryotherapy harnesses the power of cold temperatures to create well-defined zones of freezing and thawing, damaging cancerous cells and fostering cell death. In retinoblastoma, this approach focuses on small or medium-sized tumors located in the peripheral retina. Because cryotherapy can be applied repeatedly with minimal harm to surrounding healthy tissue, it is often considered both an effective and child-friendly procedure.
Historical Context and Evolution
- Early Investigations: The use of cold therapy in ophthalmology dates back to the mid-20th century, initially explored for retinal detachments. As understanding of retinoblastoma grew, clinicians tested cryotherapy for eradicating or reducing tumor growth.
- Advancements in Equipment: Over the years, cryoprobes have become more precise, enabling ophthalmologists to pinpoint the exact location of a tumor with minimal collateral damage.
- Integration with Modern Treatments: Cryotherapy today is seldom used in isolation. It is frequently paired with chemotherapy, radiation therapy, or even laser-based methods to achieve comprehensive tumor control.
Distinguishing Cryotherapy from Other Options
While various modalities exist for retinoblastoma therapy, cryotherapy stands out for its:
- Tissue-Specific Action: The extreme cold is localized, meaning that only the tumor and its immediate surroundings undergo freezing.
- Minimal Systemic Effects: Unlike systemic chemotherapy, cryotherapy spares the body from widespread toxicities, making it particularly suitable for children who are still developing.
- Repeatability: Cryotherapy can be performed multiple times if tumors recur or new tumors appear, offering a sustained approach to disease control without resorting to invasive surgeries like enucleation (eye removal).
Ideal Candidates
- Peripheral Small Tumors: Generally, retinoblastomas measuring up to 3 millimeters in height and 6 millimeters in basal dimension respond best to cryotherapy.
- Children with Bilateral Retinoblastoma: When both eyes are affected, cryotherapy can be used in a stepwise fashion along with other local or systemic treatments to save as much healthy retinal tissue as possible.
- Residual Tumor Cells Post-Chemotherapy: After systemic or intra-arterial chemotherapy has reduced tumor size, cryotherapy can help eradicate any residual cancerous areas.
Cryotherapy’s ability to preserve vision and eliminate smaller tumors has established it as a valuable tool in retinoblastoma management. In the sections that follow, we dig into the disease’s underlying genetics, common clinical signs, and the rationale for early detection—important factors that shape when and how cryotherapy is employed.
2. Understanding Retinoblastoma: Genetic Roots and Clinical Features
Retinoblastoma arises from mutations in the RB1 gene, a tumor suppressor gene critical for regulating cell growth within the retina. Early detection can be vital in preserving both life and vision. Grasping the disease’s etiology, risk factors, and early warning signs can illuminate why localized therapies like cryotherapy are so effective in specific cases.
Basic Epidemiology and Incidence
- Prevalence: Retinoblastoma affects approximately one in 15,000 to one in 20,000 live births globally, making it rare but still significant in pediatric oncology.
- Age of Onset: Most cases occur in children younger than five. Bilateral disease typically appears earlier, often within the first year of life.
Genetics at Play
- Heritable vs. Non-Heritable Forms: Around 40% of retinoblastoma cases are hereditary, involving a germline mutation in the RB1 gene. The remaining 60% are sporadic, with no familial transmission.
- Penetrance: In hereditary retinoblastoma, children often experience tumors in both eyes (bilateral). Some may develop multiple tumors over their lifetime.
- Implications for Counseling: Genetic testing can help guide treatment decisions and clarify future risks. Families may also receive counseling about the possibility of secondary cancers in heritable cases.
Clinical Manifestations
Early-stage retinoblastoma may present without pain or overt signs, underscoring the importance of routine pediatric eye screenings. Common indicators include:
- Leukocoria (White Pupillary Reflex): Often the first noticeable sign, sometimes detected in photographs where the pupil appears white or “cat’s-eye” like.
- Strabismus (Crossed Eyes): Tumors affecting the macular region can interfere with vision, potentially causing misalignment of the eyes.
- Poor Vision or Eye Redness: In some instances, there may be subtle changes in visual behavior or unexplained eye redness.
Disease Progression and Classification
Retinoblastoma follows specific growth patterns:
- Endophytic Growth: Tumor cells project into the vitreous cavity, risking seeding throughout the eye.
- Exophytic Growth: Tumor cells expand beneath the retina, potentially causing a secondary retinal detachment.
- Diffuse Infiltrating Retinoblastoma: A rare form where the tumor spreads flatly along the retina rather than forming a distinct mass.
The International Classification of Retinoblastoma system (Groups A to E) categorizes tumors based on size, location, and the presence of seeding or additional complications. Cryotherapy is more commonly used for smaller, peripheral tumors (Groups A to C), though it can serve as an adjunct for more advanced disease.
Why Early Detection Matters
- Vision Preservation: Smaller tumors are more amenable to cryotherapy, laser photocoagulation, or chemotherapy without necessitating eye removal.
- Lower Risk of Metastasis: Timely intervention in retinoblastoma typically prevents the disease from spreading beyond the eye.
- Combination Therapy Options: When identified early, patients benefit from more targeted treatments, improving overall outcomes and quality of life.
This understanding of retinoblastoma’s biology and clinical presentation lays the groundwork for appreciating how cryotherapy fits into the treatment arsenal. Next, we examine the actual freezing process—unpacking the technology that allows physicians to destroy tumors through controlled temperature manipulation.
3. The Science Behind Cryotherapy: Freezing Retinal Tumors at Their Core
At its essence, cryotherapy leverages the destructive power of extreme cold to break down malignant cells. Understanding the mechanisms by which cryotherapy targets retinoblastoma tumors sheds light on why it remains a favored approach for small tumor nodules in pediatric patients.
Mechanism of Action
- Cryoprobe Application: A specialized probe is placed on the ocular surface near or at the tumor location.
- Rapid Cooling: Using substances like nitrous oxide or carbon dioxide, the probe tip can quickly reach temperatures as low as -80°C.
- Ice Crystal Formation: Intracellular and extracellular ice crystals form in the targeted tissues.
- Cell Membrane Disruption: Repeated freeze-thaw cycles cause the cell membrane to rupture, triggering cancer cell death through both necrosis and apoptosis.
- Inflammatory Response: After thawing, the body’s inflammatory pathways clean up the destroyed cells, reinforcing tumor eradication.
Precision and Tissue Selectivity
- Localized Damage: By applying cryotherapy in a small, well-defined area, surgeons can limit the extent of damage.
- Minimal Bleeding: The vasoconstriction effect of cold reduces bleeding risk during and after the procedure.
- Repeatable Freeze-Thaw Cycles: Often, two or three cycles are administered in a single session to maximize the destructive impact on tumor cells.
Immunological and Biological Implications
Beyond direct tumor cell destruction, cryotherapy may trigger an immune response that helps the body recognize and target any remaining malignant cells. Some researchers have proposed that cryoablation can release tumor antigens, alerting the immune system to hunt for residual or microscopic cancer foci.
Cryotherapy Equipment and Advances
- Modern Cryoprobes: Smaller, more flexible devices provide enhanced control over probe placement, particularly in tricky peripheral locations.
- Real-Time Imaging: Surgeons often use indirect ophthalmoscopy or ultrasound to confirm accurate probe positioning and observe the freeze’s progress.
- Combined Modalities: Today’s pediatric oncologists may incorporate additional therapies—like thermotherapy or chemotherapy—alongside cryotherapy for synergistic tumor control.
Tumor Suitability
- Size and Location: Ideal for lesions located in the peripheral retina or near the ora serrata that measure no more than 3 mm in thickness.
- Vitreous Seeds: While cryotherapy can aid in controlling local vitreous seeds around the tumor margin, diffuse vitreous seeding may require more systemic or vitreous-targeted approaches.
When performed correctly, cryotherapy spares vital retinal structures while delivering a targeted blow to cancer cells. The following section delves into the practical execution of cryotherapy—who performs it, how often it’s given, and the pre- and post-procedure steps that ensure safety and optimal outcomes.
4. Therapeutic Journey: Protocols and Approaches for Cryotherapy
Cryotherapy for retinoblastoma is typically part of a multimodal treatment plan orchestrated by a specialized team, including pediatric oncologists, ophthalmologists, and sometimes radiation experts. The complexity of retinoblastoma warrants a highly customized approach, tailored to each child’s disease status and overall health.
Pre-Treatment Evaluation
- Imaging Studies: Fundus photography, ultrasound B-scan, and sometimes MRI help localize tumors precisely.
- Staging: Oncologists determine the retinoblastoma group classification and identify whether the tumor is unilateral (one eye) or bilateral (both eyes).
- Systemic Assessment: Children may undergo blood tests and, in some cases, bone marrow or spinal fluid evaluations if advanced disease is suspected.
Anesthesia and Sedation
Because the procedure takes place around highly sensitive ocular structures, sedation or anesthesia is commonly used:
- General Anesthesia: More common in very young children, ensuring the child remains still throughout the procedure.
- Local or Regional Blocks: In some cases, older children or those undergoing multiple treatments may receive local anesthesia with sedation.
Procedure Step-by-Step
- Positioning: The child is usually placed in a supine position with the eye clearly visible to the surgeon.
- Eye Stabilization: An eyelid speculum keeps the eye open; a specialized surgical lens or indirect ophthalmoscope helps visualize the retina.
- Probe Placement: The cryoprobe is gently placed on the sclera over the tumor site. Physicians often map out the tumor margin to ensure adequate coverage.
- Freezing Cycles: Each freeze application lasts from 20 to 40 seconds, repeated multiple times, allowing a thaw in between to maximize tumor cell destruction.
- Assessment: The surgeon may visually confirm an ice ball formation in the retina during each cycle.
Combination Therapies
- Chemoreduction: Systemic or intra-arterial chemotherapy is often used to shrink the tumor before cryotherapy. This approach also helps treat microscopic disease that might be present elsewhere in the eye.
- Thermotherapy: Laser or heat-based therapy can serve as a finishing step, consolidating tumor destruction.
- Focal Laser Photocoagulation: In some setups, photocoagulation is used for residual tumor edges post-freeze.
Frequency and Number of Sessions
The number of cryotherapy sessions needed varies. Generally:
- Tumor Response: If the lesion shrinks significantly and no new tumors appear, follow-up sessions may be minimal.
- Dormant or Recurrent Tumors: If retinoblastoma remains or resurfaces, additional cryo treatments might be performed.
- Follow-Up: Comprehensive eye exams every few weeks or months help detect new lesions, especially in hereditary cases.
Supportive Care and Monitoring
Post-treatment follow-up focuses on:
- Visual Acuity Assessments: Tracking the child’s vision as they age.
- Secondary Cancer Risks: In heritable cases, there is a lifelong risk of additional malignancies, making regular checkups essential.
- Psychosocial Support: Families often benefit from counseling or support groups, given the emotional toll of cancer treatment in young children.
Equipped with these protocols, cryotherapy stands as a highly adaptable, child-centered solution. Its true impact, however, is best measured by tangible outcomes—how well it annihilates tumors, preserves sight, and avoids complications. We turn next to an in-depth look at cryotherapy’s safety record and therapeutic success rates.
5. Safety and Efficacy of Cryotherapy in Pediatric Oncology
Cryotherapy has been a mainstay in retinoblastoma treatment for decades, yet ongoing research and clinical reports continue to affirm its significance. With careful patient selection and experienced surgical technique, cryotherapy yields impressive tumor control rates while limiting damage to surrounding tissues. Still, as with any cancer therapy, it carries inherent risks that must be managed.
Established Effectiveness
- Local Tumor Control: Numerous studies indicate that 70% to 90% of small peripheral retinoblastomas treated with cryotherapy achieve complete local control.
- Vision Preservation: Because cryotherapy is minimally invasive in terms of ocular structures, many children retain functional vision in the treated eye(s).
- Reduced Need for Enucleation: By controlling small tumors, cryotherapy can spare patients from radical surgery such as eye removal, often improving psychosocial outcomes.
Adverse Effects and Complications
- Inflammation and Edema: The freeze-thaw cycle prompts a localized inflammatory response, causing mild swelling or discomfort. These symptoms usually subside within days.
- Retinal Pigment Epithelium Changes: The treated area may show pigmentation changes, rarely impacting central vision if the tumor lies in the periphery.
- Perforation Risks: Extremely rare, but improper technique or excessive freeze time could theoretically damage deeper ocular layers.
- Recurrence: Tumors can recur if not fully treated or if new tumor foci develop in patients with hereditary retinoblastoma, requiring multiple treatment sessions.
Comparative Safety with Other Treatments
When assessing cryotherapy’s safety relative to other localized interventions:
- Laser Photocoagulation: Similar in terms of local control for small tumors, though laser therapy requires a clear media and precise focus on the tumor surface.
- Plaque Radiotherapy: Offers deeper tissue penetration but carries a risk of radiation-induced changes, including potential damage to optic nerve or lens.
- Systemic or Intra-Arterial Chemotherapy: Targets multiple lesions and any microscopic spread but often involves systemic toxicities, from immune suppression to hearing loss (in the case of certain agents).
Long-Term Outcomes
Overall survival rates in retinoblastoma exceed 95% in developed regions, primarily due to advanced detection and multimodal therapy that includes cryotherapy. From a functional standpoint, many survivors benefit from preserved ocular structures, with minimal risk of secondary complications from cryotherapy alone.
Safety Protocols
- Surgeon Training: Cryotherapy demands a deep understanding of ocular anatomy and technique to target the tumor accurately.
- Patient Monitoring: Pediatric oncologists and ophthalmologists conduct ongoing eye exams to track any late complications such as scarring near critical visual pathways.
- Concomitant Strategies: Frequent imaging and adjunct therapies can rapidly address incomplete tumor response, minimizing the chance of progression or metastasis.
These safety and efficacy metrics underscore cryotherapy’s place as a gold standard for smaller retinoblastomas. However, treatment strategies do not remain stagnant. Scientific inquiries drive refinements in cryoprobe technology, patient selection criteria, and combined treatment modalities—topics we explore next when detailing the latest in clinical research.
6. Emerging Studies and Clinical Progress: Latest Research Insights
Scientific exploration in the realm of retinoblastoma therapy rarely stands still. Across the globe, medical researchers are investigating ways to optimize cryotherapy outcomes, reduce complications, and integrate it more seamlessly with cutting-edge therapies. Below are some recent findings and ongoing investigations that illuminate the future of cryotherapy in pediatric oncology.
Novel Techniques and Device Enhancements
- Micro-Cryoprobes: Recent prototypes offer even more precision, with smaller probe tips for pinpointing tiny tumors in challenging peripheral locations. Preliminary data suggest improved lesion targeting and potentially fewer sessions per patient.
- Smart Temperature Monitoring: Some cryotherapy units now include digital feedback loops, allowing surgeons to maintain consistent temperatures and reduce the risk of overtreatment.
Combination Therapies in Focus
- Chemotherapy Plus Cryotherapy: A study published in the American Journal of Ophthalmology analyzed children receiving systemic chemoreduction for bilateral retinoblastoma. Over 80% of residual or recurrent peripheral tumors responded to cryotherapy, highlighting synergy between the two approaches.
- Immunotherapy Adjuncts: Preliminary lab-based research indicates that cryotherapy-induced cell death might present tumor antigens to the immune system. Future trials may investigate combining cryoablation with immunotherapeutic agents to curb microscopic tumor spread.
Early Detection and Genetic Testing
- Advanced Screening Protocols: Genetic screening for RB1 mutations can identify at-risk infants. Earlier detection of lesions (even subclinical microtumors) opens the door for more targeted cryotherapy, often yielding excellent outcomes.
- Liquid Biopsy: Though still under investigation, some centers explore analyzing circulating tumor DNA to monitor therapy response. This could allow for real-time adjustments to cryotherapy plans.
Longitudinal Studies on Survivorship
- Vision and Quality of Life: Multi-year follow-ups in retinoblastoma survivors confirm that timely cryotherapy, paired with systemic or localized treatments, correlates with stable or improved vision over time.
- Psychosocial Parameters: Data also show that families value cryotherapy for minimizing invasive procedures. Reduced reliance on external-beam radiation, which poses long-term risks, improves the child’s quality of life during and after cancer therapy.
Global Outreach and Accessibility
- Resource-Constrained Settings: In regions lacking advanced radiation units, cryotherapy can be a cost-effective local therapy. Recent philanthropic projects train local ophthalmologists in cryotherapy application, potentially expanding care access in low- and middle-income countries.
- Telemedicine and Collaborative Networks: Partnerships between specialized cancer centers and regional hospitals have led to shared protocols, enabling earlier referrals and improved patient outcomes.
The overarching trend is clear: cryotherapy maintains a pivotal role in retinoblastoma treatment, continually enhanced by technological, genetic, and systemic therapy innovations. As research and clinical collaboration grow, the therapy is poised to become even more refined, effective, and accessible, offering hope for children worldwide facing retinoblastoma diagnoses.
7. Weighing the Costs: Therapy Price and Financial Factors
Cryotherapy for retinoblastoma involves specialized equipment and expert clinical oversight, often making the cost higher than standard outpatient procedures. Typical prices can range from $1,000 to $3,500 per session in centers that routinely handle pediatric oncology cases. Higher-end facilities might charge more, especially if advanced imaging, anesthesia, and follow-up exams are bundled into the final bill. Insurance coverage often offsets a significant portion of these costs when retinoblastoma is diagnosed, given that preserving vision and life takes priority in pediatric cancer care. For families without comprehensive insurance, some hospitals may offer financial assistance or philanthropy-driven support programs that help manage the out-of-pocket expenses. Ultimately, exact fees depend on factors such as geographic location, the child’s specific treatment plan, and whether combination therapies like chemotherapy or laser photocoagulation are required.
Disclaimer:
This article is intended for informational purposes only and does not replace professional medical consultation. Always seek the advice of a qualified healthcare provider regarding any questions about a medical condition or treatment.
We encourage you to share this article with friends, family, or on social media platforms like Facebook and X (formerly Twitter). By spreading knowledge about cryotherapy for retinoblastoma, you can help other families navigate treatment options, connect with medical experts, and find the best possible care for their children. Feel free to use any share buttons or methods you prefer—together, we can foster greater awareness and hope for those confronting pediatric eye tumors.