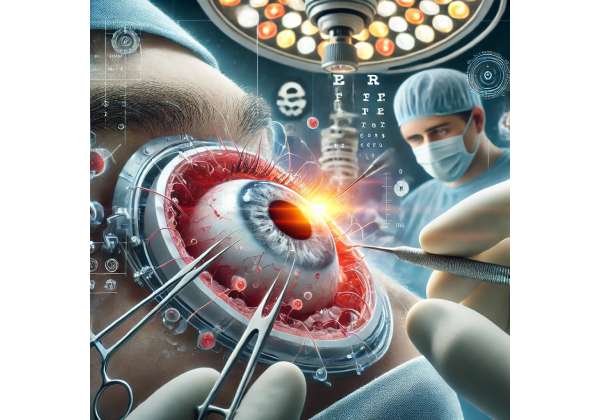
Have you ever imagined a treatment that can promote natural corneal healing by harnessing the body’s own regenerative abilities? Umbilical cord-derived stem cells are emerging as a powerful ally in restoring clarity to damaged corneas, offering hope for those recovering from ocular trauma. Rather than relying solely on conventional transplant methods or extended use of artificial ocular devices, this innovative approach seeks to rejuvenate the cornea at a cellular level. By delivering potent stem cells that can differentiate into healthy corneal cells, the therapy aims to accelerate recovery times and reduce complications.
One of the most compelling aspects of stem cell-based corneal repair is that it potentially provides a more stable and holistic approach compared to standard treatments. Many eye specialists look forward to a time when corneal burns, lacerations, or perforations can be managed with fewer invasive procedures—relying instead on regenerative medicine to foster long-lasting improvements in vision. Especially for individuals who might otherwise face lengthy waitlists for donor tissue or inconsistent healing from grafts, umbilical cord-derived stem cells present an appealing alternative.
Below, we take a deep dive into how umbilical cord stem cells are cultivated and employed for corneal repair, the protocols guiding their use, key clinical trials and findings, safety measures, and a look at associated costs. By understanding the multifaceted world of this evolving therapy, patients and practitioners alike can better appreciate the scope and promise of regenerative eye care.
Deeper Look at Umbilical Cord-Derived Stem Cell Therapy for Corneal Injuries
Umbilical cord-derived stem cells, sometimes referred to as UC-MSCs (umbilical cord mesenchymal stem cells), have steadily gained recognition in medical circles for their versatility and potent regenerative capacity. Although they first entered the broader research scene through orthopedic and systemic disease applications, these cells are increasingly drawing attention in ophthalmology. The unique combination of anti-inflammatory properties, robust differentiation potential, and relatively easy sourcing from donated umbilical cords is propelling them into the spotlight for corneal healing.
What Sets These Stem Cells Apart
When it comes to repairing a delicate structure like the cornea, not all stem cells are created equal. Several qualities make umbilical cord-derived cells highly appealing:
- Low Immunogenicity: The cornea itself is considered “immune privileged” in many respects, but using cells that elicit minimal immune responses further reduces the risk of rejection. UC-MSCs naturally have a lower likelihood of triggering adverse immune reactions.
- Anti-Inflammatory Effects: Inflammation often complicates corneal healing, especially after trauma. Studies suggest that UC-MSCs can modulate inflammatory processes in the eye, decreasing the likelihood of scarring or additional damage.
- High Multipotency: These cells have the ability to differentiate into various cell types, including those resembling corneal epithelial cells. This allows the therapy to target the actual site of injury and produce cells that integrate more seamlessly into the corneal structure.
- Ethical and Abundant Source: Umbilical cords are typically discarded after birth, so harvesting stem cells from this tissue can be done without harming newborns or mothers. This approach bypasses many ethical questions that arise with embryonic stem cells, while providing an abundant, renewable source of therapeutic material.
Why the Cornea Needs Special Attention
The cornea, a transparent, dome-shaped surface covering the eye, is essential for refracting light and maintaining clear vision. Any damage from trauma—chemical burns, blunt force, sharp objects—can significantly impair eyesight. Traditional treatments include sutures, grafts from donor tissue, or corneal transplants if severe damage exists. While these methods are often successful, they come with notable limitations and risks:
- Donor Shortages: Accessing suitable donor corneas isn’t always guaranteed, leading to waiting lists.
- Risk of Rejection: Even with immune-suppressing regimens, donor grafts can be rejected.
- Slow Recovery and Scarring: After corneal transplants, patients may face an extended healing timeline and potential scarring, impacting final visual outcomes.
By introducing umbilical cord-derived stem cells, ophthalmologists aim to shorten recovery windows, minimize complications, and improve the likelihood of fully restoring corneal clarity. The notion is that these stem cells don’t just patch up the damage but actively prompt the body to create new, healthy tissue.
Mechanisms Underlying Regeneration
While the exact biological pathways remain a subject of ongoing study, researchers have pinpointed several potential mechanisms by which UC-MSCs facilitate corneal repair:
- Paracrine Activity: The stem cells release growth factors and signaling molecules that direct local tissue to regenerate, encouraging proliferation of native corneal cells.
- Direct Differentiation: A fraction of the transplanted stem cells may themselves become corneal epithelial or stromal cells, integrating into the eye’s surface and stroma.
- Reduction in Fibrosis: Collagen deposition and scar formation are hallmarks of corneal injury. UC-MSCs may slow or prevent these processes, keeping the cornea more transparent.
- Enhanced Neovascularization Control: A well-regulated blood supply is crucial for tissue healing, but too much vessel growth can obscure vision. Stem cells may help moderate abnormal vessel formation during corneal repair.
Though these mechanisms are complex, the end result is a multifaceted boost to the body’s innate repair capabilities. Rather than relying on a single process or medication, UC-MSCs tackle various factors that hamper corneal healing, from persistent inflammation to insufficient cell replacement.
Potential Roles Beyond Trauma
Although the primary spotlight is on how umbilical cord-derived stem cells can mend trauma-induced corneal injuries, there’s speculation about broader applications. Conditions such as corneal ulcers, persistent epithelial defects, and certain degenerative disorders might benefit from the therapy’s regenerative properties as well. In these contexts, harnessing stem cells could spare patients from repeated graft procedures or long-term immunosuppression.
Moreover, some scientists are exploring how these cells might work alongside other cutting-edge technologies. For instance, combining bioengineered scaffolds with UC-MSCs in a lab setting may open up possibilities for custom-tailored corneal implants. If proven safe and effective, these advanced solutions would represent a monumental leap in personalized ocular medicine.
Ethical and Logistic Considerations
One of the striking advantages of umbilical cord-derived cells is that they sidestep many ethical debates. Parents can donate umbilical cords post-birth, ensuring a consistent supply of stem cells that doesn’t rely on fetal or embryonic sources. Additionally, processing and storing these cells is relatively straightforward once the protocols are established. That said, ensuring quality control and standardization across different cell banks and clinics is vital. Regulatory oversight is crucial to prevent subpar or contaminated cell sources from reaching patients.
Logistics also play a role in how quickly this therapy can be scaled. Freezing and shipping UC-MSCs must adhere to strict guidelines to preserve viability. As more specialized centers adopt the approach, local availability of cryopreserved, high-quality stem cells could expedite treatment for acute corneal injuries.
Looking Ahead
While numerous case reports and preliminary trials promise encouraging outcomes, the field is still evolving. Long-term follow-ups and large-scale randomized studies remain essential to confirm that the therapy consistently provides enduring corneal transparency and stable visual acuity. Nonetheless, the early results show that umbilical cord-derived stem cell therapy stands as a robust and forward-thinking option in the realm of corneal regeneration.
As the science matures, patients who once faced daunting prognoses—such as progressive vision loss or repeated transplant failures—may discover a renewed sense of optimism. Beyond alleviating the immediate physical toll of ocular trauma, a successful regenerative approach can restore independence, reduce healthcare burdens, and enhance overall quality of life.
Step-by-Step Approaches and Treatment Procedures
While scientific breakthroughs often make headlines, practical guidance is key to understanding how these therapies move from lab to clinic. In the case of umbilical cord-derived stem cells for corneal repair, protocols aim to optimize safety, precision, and post-treatment follow-up. Below, we walk through the typical steps patients and providers might encounter when considering or undergoing this therapy.
Patient Evaluation and Candidacy
Before any procedure takes place, the clinical team conducts a detailed assessment of the injury and overall ocular health. This often includes:
- Comprehensive Eye Examination: Checking visual acuity, corneal thickness, topography (mapping the surface), and looking for any signs of infection or uncontrolled inflammation.
- Medical History Review: Patients with autoimmune conditions, severe diabetes, or systemic infections might need special precautions.
- Extent of Corneal Damage: Not all corneal traumas are alike. For moderate to severe injuries that affect large areas or deeper layers, umbilical cord stem cell therapy might prove especially beneficial.
In some cases, doctors might prefer to stabilize the injury first using antibiotics, anti-inflammatory drugs, or traditional surgery—particularly if there’s an active infection or perforation.
Harvesting and Preparing Stem Cells
One reason umbilical cord-derived stem cells stand out is that they’re typically ready for use via established stem cell banks or specialized tissue facilities. Donors are screened rigorously to ensure the stem cells are free of infections and genetically stable. Once harvested, the cells undergo:
- Isolation and Expansion: Technicians culture UC-MSCs in controlled lab conditions, encouraging them to multiply while maintaining their therapeutic properties.
- Quality Control Checks: Tests confirm that the cells remain uncontaminated, genetically healthy, and have not differentiated prematurely.
- Cryopreservation: For ease of shipment and storage, the final product can be frozen in liquid nitrogen, ready to be thawed at the point of use.
Medical teams coordinate with the cell bank to schedule a timely delivery, ensuring the cells are viable when the patient is prepared for treatment.
Administration Techniques
Delivery of UC-MSCs to the cornea can vary, but a few common methods have emerged:
- Topical Application: In milder injuries confined to the epithelium, clinicians might place a high concentration of stem cells in drop form or via a specialized membrane contact lens. This approach is less invasive but might be less effective for deeper trauma.
- Intrastromal Injection: For more substantial corneal damage, surgeons use a fine needle to inject stem cells into the mid or anterior stroma, closer to the site of injury. This ensures that a higher number of cells interact directly with the damaged area.
- Amniotic Membrane Grafts Loaded with Stem Cells: Sometimes, an amniotic membrane (obtained from the inner layer of the placenta) is used as a scaffold. It’s soaked with UC-MSCs and placed over the cornea to foster localized regeneration.
All these techniques aim to create an environment where the stem cells can remain in contact with the wound site long enough to exert their therapeutic effects. Depending on the severity of trauma, the doctor may combine techniques—for example, injecting some cells while also applying them topically to enhance coverage.
Immediate Post-Procedure Care
Once the cells are administered, it’s crucial to protect the eye and support the healing process:
- Protective Shields or Bandage Lenses: Covering the eye can shield it from external irritants, light sensitivity, and accidental rubbing.
- Prescribed Medications: Antibiotic eye drops may prevent infection, while mild anti-inflammatories can keep excessive swelling in check without negating the stem cells’ benefits.
- Monitoring Eye Pressure: Some individuals may be at risk for ocular hypertension or other pressure anomalies, so routine checks are advised in the initial weeks.
Patients are typically instructed to avoid strenuous activities, heavy lifting, or environments with high contamination risk (such as dusty worksites). Regular follow-up appointments, possibly within the first week, then monthly, help doctors assess epithelial healing, measure visual acuity, and confirm that no complications like secondary infection have arisen.
Follow-Up and Long-Term Monitoring
One of the goals of corneal regeneration therapy is to achieve stable and lasting improvements. Thus, monitoring the cornea’s response is essential:
- Repeat Imaging: Techniques like corneal topography or optical coherence tomography (OCT) track changes in corneal thickness and smoothness.
- Visual Function Tests: Beyond the classic eye chart, contrast sensitivity exams or glare tests can reveal subtle gains in functional vision.
- Periodic Inflammation Checks: In some cases, low-grade inflammation might flare up months later, requiring prompt management to preserve progress.
- Retreatment Strategies: If the cornea doesn’t achieve the desired healing, or partial regression occurs, additional stem cell applications could be considered.
For many patients, the first few months after therapy represent a crucial window when the newly introduced stem cells integrate with the tissue. Provided healing continues positively, check-ups may become less frequent over time, settling into a standard annual eye exam once stability is confirmed.
Potential Adjunctive Therapies
Given the cornea’s delicate nature, doctors might complement stem cell therapy with other supportive approaches:
- Nutritional Supplements: Vitamins A and C, alongside specific amino acids, are sometimes recommended to fuel regenerative processes.
- Low-Level Laser Therapy: Some preliminary studies hint that certain noninvasive laser modalities can stimulate cell activity, although data remains limited.
- Physical Shields or Protective Goggles: Especially for individuals exposed to harsh UV rays or physically risky environments, wearing goggles can shield the healing tissue from microtraumas.
Where indicated, these adjuncts can accelerate corneal restoration, preventing setbacks. With careful adherence to the outlined protocols, patients often see improvements in corneal clarity over the subsequent weeks and months.
Continual Advances in Protocols
As with any emerging field, the best-practice standards for umbilical cord-derived stem cell therapy in corneal repair keep evolving. Rigorous clinical trials refine dosing, delivery, and aftercare techniques. Over time, more refined strategies—like combining growth factors or using genetically modified cells for better survival rates—could reshape how this therapy is performed.
In the meantime, the structured approach of thorough patient evaluation, standardized cell preparation, targeted application, and close follow-up yields encouraging outcomes in many pilot programs and early clinical experiences. Patients able to receive this innovative treatment often discover a shorter healing curve, lower complication rates, and an enhanced quality of vision relative to older surgical options.
Notable Clinical Investigations and Observational Findings
The rise in interest surrounding umbilical cord-derived stem cells for corneal repair has spurred an uptick in clinical trials, peer-reviewed studies, and case reports. While results can vary by methodology and patient populations, they consistently point toward a capacity for faster healing and improved optical outcomes. Below are highlights from key research that underline both the promise and the challenges involved in translating this therapy into mainstream practice.
Early Lab-Based Breakthroughs
Pioneering efforts to confirm UC-MSC efficacy began in controlled laboratory environments. For instance, cell culture and animal models allowed researchers to observe whether these stem cells could truly restore damaged corneal cells. Published findings in the Journal of Ocular Tissue Science (2015) showed:
- Encouraging Cell Integration: UC-MSCs co-cultured with corneal epithelial cells displayed robust growth patterns and adhered well, indicative of a supportive microenvironment.
- Reduced Pro-Inflammatory Markers: Levels of cytokines responsible for excessive scarring dropped in cell cultures treated with stem cells compared to controls.
Such data reinforced the notion that UC-MSCs are more than a simple bandage. They appear to orchestrate multiple pathways essential for natural, scar-free healing.
Translational Animal Studies
After validating effects in cell cultures, the next step typically involved induced corneal injury in animal models—rabbits, rodents, or pigs. A landmark 2018 study in the International Journal of Experimental Ophthalmology featured rabbits with deep corneal wounds:
- Faster Closure of Defects: By the second week, nearly 80% of wounds in the UC-MSC-treated group had re-epithelialized compared to 50% in the control group receiving conventional antibiotic and steroid drops alone.
- Clearer Visual Outcomes: Investigators documented less haziness on slit-lamp exams, suggesting fewer collagen deposits typical of scarring.
- Minimal Adverse Reactions: No severe graft-versus-host-like symptoms or other major inflammatory responses were observed.
These findings were instrumental in propelling human trials forward. Demonstrating that UC-MSCs could accelerate repair in a species with an eye anatomy somewhat similar to humans heightened optimism.
Controlled Human Trials
Studies involving human subjects are the gold standard, offering a deeper glimpse into clinical value. While still somewhat limited in number, several small-scale trials provide compelling evidence. A 2020 pilot study published in Clinical Ophthalmic Regeneration examined 15 patients with moderate corneal trauma. They received topical UC-MSC therapy for four weeks, monitored against a control group of 10 individuals receiving typical medical management. Key takeaways included:
- Improved Epithelial Integrity: Patients on UC-MSC therapy demonstrated faster closure of epithelial defects and fewer signs of corneal haze.
- Better Visual Acuity: On average, the UC-MSC group improved 2–3 Snellen lines more than controls. Some even regained near-normal clarity.
- No Serious Adverse Effects: Mild irritation or redness was noted in a few cases, but these resolved quickly with artificial tears.
Though modest in scale, the trial’s results stirred excitement, showcasing how a relatively noninvasive regimen might significantly alter recovery trajectories for traumatic corneal injuries.
Observational Case Reports
Beyond formal trials, real-world case reports can illustrate nuanced outcomes. A 2021 article in the Global Stem Cell Ophthalmology Journal described a 42-year-old patient who sustained a chemical burn to the right eye, leading to persistent epithelial defects. After failing to respond adequately to grafts and standard medication, the patient was administered a combination of intrastromal UC-MSC injections and amniotic membrane overlay. Within six weeks:
- Healing of Epithelial Defects: Slit-lamp evaluations confirmed complete re-epithelialization and significantly reduced corneal opacity.
- Marked Symptom Relief: The patient reported less pain, photophobia, and dryness as healing progressed.
- Stable Result at 6-Month Checkup: Follow-up revealed a stable epithelial surface with only minimal scarring.
Though anecdotal, such reports highlight the therapy’s potential to salvage vision even in severe, treatment-resistant scenarios. They also underscore the role of combining UC-MSCs with other supportive materials like amniotic membranes.
Comparisons with Conventional Interventions
Directly pitting stem cell therapy against standard corneal transplants or donor grafts can be challenging—each approach fits different contexts. Nevertheless, preliminary comparative analyses hint that:
- Reduced Need for Transplants: Some patients who might otherwise require a partial or full-thickness transplant could forego it if the corneal stroma responds well to UC-MSC therapy.
- Faster Rehabilitation: Individuals recover function more quickly, resuming daily activities with fewer postoperative restrictions.
- Lower Immunosuppressant Dependency: The immunomodulatory nature of UC-MSCs often translates to less reliance on potent drugs.
That said, for extensive corneal losses or advanced diseases with near-total structural compromise, a transplant may remain unavoidable. Stem cells may complement the transplant, rather than fully replace it in such advanced situations.
Remaining Hurdles and Future Directions
Despite promising results, not all studies conclude with unequivocal success. Some emphasize that the longevity of UC-MSC integration or the durability of visual gains beyond the first year is still uncertain. Larger, randomized controlled trials with broader patient demographics are needed to validate initial findings. Other points needing clarification include:
- Optimized Dosages and Frequency: Research is ongoing to determine how many cells are optimal, how often they should be applied, and whether booster treatments are beneficial.
- Long-Term Safety: While short-term complications appear minimal, understanding the risk of ectopic tissue growth or late-onset inflammation is essential.
- Cost-Effectiveness: Widespread adoption hinges on whether this therapy is economically sustainable compared to conventional graft-based interventions.
Nevertheless, the overall scientific conversation tilts toward enthusiasm. Over the next decade, improved standardization of UC-MSC processing, robust multi-center trials, and refined application methods will likely cement its status as a major player in corneal trauma management.
By systematically analyzing lab, animal, and human data sets, the ophthalmic community is piece by piece constructing a solid foundation for umbilical cord-derived stem cell therapy. While it is no silver bullet, its capacity to stimulate natural corneal regeneration without severe side effects or complex ethical obstacles is undeniably ground-breaking in modern eye care.
Assessing Efficacy and Precautions in Stem Cell Procedures
Umbilical cord-derived stem cell therapy consistently showcases potential for speeding up corneal healing and enhancing final visual outcomes, especially in cases of ocular trauma. Many patients experience more comfortable recoveries, reduced inflammation, and fewer complications compared to traditional interventions alone. However, as with any emerging medical procedure, it is not free of risks.
Minor side effects typically revolve around localized eye irritation or mild redness, often short-lived and manageable with over-the-counter lubricating drops. Invasive techniques, such as intrastromal injections, carry a small risk of infection, corneal perforation, or elevated intraocular pressure. Strict sterile protocols and meticulous follow-up significantly reduce these issues. Thus far, large-scale inflammatory or rejection-related complications appear less common than in donor tissue transplants, thanks to the immunoprivileged status of the cornea and the low immunogenicity of UC-MSCs.
It is important for patients to undergo thorough evaluations to identify any underlying conditions—such as active infection, severe uncontrolled autoimmune disease, or advanced corneal thinning—that might complicate stem cell therapy. In some instances, doctors may recommend partial stabilization of the injury via antibiotic regimens or minor surgical interventions before moving forward with cell-based treatments. Transparent communication, realistic expectations, and adherence to post-procedure guidelines all help ensure safer, more predictable outcomes.
Price Outlook for Umbilical Cord-Derived Stem Cell Therapy
Costs for this regenerative approach differ widely, influenced by factors like geographic location, the specific technique used, and whether the cells come from private or public stem cell banks. Typical estimates in regions where the therapy is available can range between \$2,500 and \$5,000 per treatment session, although packages that include follow-up visits and additional materials may drive the price higher. Insurance coverage for stem cell-based eye procedures remains inconsistent, so patients often bear at least part of the expense themselves.
This information is offered solely for educational purposes and does not replace a personalized consultation with a qualified medical professional. If you believe that umbilical cord-derived stem cell therapy could aid in your corneal recovery or are simply intrigued by its potential, consider reaching out to an eye care specialist for individualized guidance. Feel free to share this article on Facebook, X (formerly Twitter), or any platform you prefer—spreading the word can inspire hope and enlighten others about this groundbreaking path to restoring clearer vision.