Congenital Retinal Dystrophies Basics
Congenital retinal dystrophies are a group of inherited disorders that affect the retina, the light-sensitive layer at the back of the eye, beginning at birth. These conditions are distinguished by progressive degeneration of retinal cells, which results in impaired vision and, in severe cases, total blindness. The onset of symptoms varies, but they usually appear early in life, often in infancy or childhood. Understanding congenital retinal dystrophies is critical for early detection and intervention, which can help patients manage symptoms and improve their quality of life. These disorders are genetically diverse, with mutations in various genes that control retinal function and health.
Exploring Congenital Retinal Dystrophies
Congenital retinal dystrophies are a diverse group of conditions, each with unique genetic, clinical, and pathological characteristics. These disorders are primarily inherited in an autosomal recessive, autosomal dominant, or X-linked manner, so genetic counseling is an important part of managing them.
Etiology and Pathophysiology
The retina is made up of multiple layers of cells, including photoreceptors (rods and cones), bipolar cells, ganglion cells, and supporting glial cells. Congenital retinal dystrophies are characterized by the degeneration of photoreceptors or other retinal cells as a result of genetic mutations that impair development, function, or survival.
Rod-Cone Dystrophies
Rod-cone dystrophies are a type of congenital retinal dystrophy, the most common of which is retinitis pigmentosa (RP). RP is defined by the loss of rod photoreceptors, which are responsible for peripheral and night vision, followed by cone photoreceptor degeneration, which affects central and color vision. Mutations in more than 60 genes have been linked to RP, including those involved in phototransduction, the visual cycle, and retinal cell structure.
Cone-Rod Dystrophies
Cone-rod dystrophies (CRD) are another important group in which cone photoreceptors degenerate first, resulting in early loss of central and color vision, followed by rod photoreceptor degeneration. CRDs are associated with mutations in genes such as ABCA4, RIM1, and CRX, which are required for cone function and maintenance.
Leber’s Congenital Amaurosis
Leber congenital amaurosis (LCA) is a severe form of congenital retinal dystrophy that frequently causes profound vision loss or blindness at birth or in early infancy. LCA is genetically heterogeneous, with mutations found in over 20 genes, including RPE65, CEP290, and GUCY2D. These genes are involved in a variety of retinal processes, including photoreceptor development, the visual cycle, and RPE function.
Stargardt’s Disease
Stargardt disease, also known as juvenile macular degeneration, is a type of congenital retinal dystrophy that causes progressive loss of central vision due to the accumulation of lipofuscin pigments in the RPE. The most commonly associated gene with Stargardt disease is ABCA4, which encodes a protein involved in the visual cycle and retinal molecule transport.
Clinical Presentation
Clinical manifestations of congenital retinal dystrophies vary greatly depending on the disorder and genes involved. Common symptoms include:
- Night Blindness: Often one of the first symptoms, especially in rod-cone dystrophies like retinitis pigmentosa.
- Peripheral Vision Loss: Gradual narrowing of the visual field, resulting in “tunnel vision.”
- Central Vision Loss: Common in cone-rod dystrophies and conditions such as Stargardt disease, which impairs reading, face recognition, and other detailed visual tasks.
- Photophobia: Increased light sensitivity, which is common in conditions that affect cone photoreceptors.
- Nystagmus: Involuntary eye movements that are frequently seen in severe forms such as Leber congenital amaurosis.
- Color Vision Deficiency: Poor color discrimination, especially in cone-rod dystrophies.
Gene Diversity and Inheritance Patterns
Congenital retinal dystrophies exhibit significant genetic heterogeneity, with each condition potentially caused by mutations in a variety of genes. This genetic diversity complicates the diagnosis, necessitating extensive genetic testing to determine the underlying cause.
Autosomal Recessive Inheritance
Many congenital retinal dystrophies, such as LCA and Stargardt disease, have an autosomal recessive inheritance pattern. Affected individuals inherit two copies of the mutated gene, one from each parent, and are usually carriers with no symptoms.
Autosomal Dominant Inheritance
Some forms of retinitis pigmentosa and other dystrophies are autosomal dominant, meaning that a single copy of the mutated gene from an affected parent is enough to cause the disease.
X-Linked Inheritance
X-linked congenital retinal dystrophies, such as X-linked retinitis pigmentosa, are caused by X-chromosome gene mutations. Males are more severely affected because they have only one X chromosome, whereas females may be carriers and experience milder symptoms.
Effects on Quality of Life
Congenital retinal dystrophies cause progressive vision loss, which has a significant impact on one’s quality of life. Children with these conditions may struggle in school and require special accommodations and support. As the disease progresses, people may experience difficulties with daily activities, mobility, and independence, necessitating the use of assistive devices and rehabilitation services.
Prevention Tips
- Genetic Counseling and Testing: Couples with a family history of congenital retinal dystrophies should seek genetic counseling before becoming pregnant. Early genetic testing can help identify carriers and estimate the risk of having affected offspring.
- Prenatal Screening: Advances in prenatal genetic screening have enabled the early detection of retinal dystrophies in utero. Early diagnosis allows for more timely interventions and planning for specialized care.
- Regular Eye Examinations: Routine eye exams, beginning in infancy, are critical for early detection of retinal dystrophies. Early detection can lead to prompt treatment, potentially slowing disease progression and preserving vision.
- Healthy Lifestyle Choices: Eating a healthy diet high in antioxidants, vitamins, and minerals is important for eye health because it supports overall retinal function and may slow disease progression.
- Avoiding Excessive Sunlight Exposure: Wearing sunglasses and hats to protect the eyes from harmful UV radiation can help to prevent retinal damage and promote long-term eye health.
- Awareness and Education: Informing families about the signs and symptoms of congenital retinal dystrophies can lead to earlier diagnosis and intervention, resulting in better outcomes for affected individuals.
- Participation in Clinical Trials: Enrolling in clinical trials for emerging therapies can give you access to cutting-edge treatments while also helping to advance research in congenital retinal dystrophies.
- Support Networks: Joining a support group or network for people with congenital retinal dystrophies can provide emotional support, resources, and information on how to effectively manage the condition.
Diagnostic methods
Congenital retinal dystrophies must be diagnosed using a comprehensive approach that combines clinical evaluation and advanced diagnostic techniques to accurately identify the underlying genetic mutations and assess the extent of retinal damage.
Clinical Examination
The diagnostic process begins with an ophthalmologist performing a thorough clinical examination. This includes a detailed patient history, with a focus on symptom onset and progression, as well as a family history to identify any hereditary patterns. Visual acuity tests are used to determine the extent of vision loss.
Fundus Examination
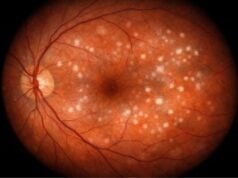
A fundus examination with ophthalmoscopy allows the ophthalmologist to see the retina directly. Pigmentary deposits, retinal thinning, and atrophy can all be signs of retinitis pigmentosa, a type of retinal dystrophy.
Electroretinography (ERG)
Electroretinography (ERG) is a critical diagnostic tool that assesses the electrical responses of different retinal cells (rods, cones, and inner retinal cells) to light. ERG can help distinguish between rod-cone and cone-rod dystrophies by revealing distinct patterns of retinal dysfunction.
Optical Coherence Tomography(OCT)
Optical coherence tomography (OCT) generates high-resolution cross-sectional images of the retina, allowing for precise visualization of retinal layers. OCT can detect structural abnormalities like photoreceptor layer thinning, retinal pigment epithelium (RPE) changes, and macular abnormalities, which are common in congenital retinal dystrophies.
Fundus Autofluorescence (FAF)
Fundus autofluorescence (FAF) imaging is used to evaluate the health of the RPE by detecting lipofuscin, a byproduct of photoreceptor turnover. Increased autofluorescence can reveal areas of retinal stress or degeneration, assisting in mapping the extent of retinal damage.
Genetic Testing
Genetic testing is required to confirm the diagnosis of congenital retinal dystrophies. Advances in next-generation sequencing (NGS) have enabled the identification of specific genetic mutations linked to these disorders. Comprehensive genetic panels can screen for mutations in multiple genes at the same time, providing a precise molecular diagnosis while also informing prognosis and potential treatment options.
Innovative Diagnostic Techniques
Recent advances in diagnostic imaging and molecular genetics have improved our ability to accurately diagnose congenital retinal dystrophies. Adaptive optics (AO) imaging provides ultra-high-resolution images of photoreceptors, while whole exome sequencing (WES) provides a more comprehensive genetic analysis. These cutting-edge techniques enable early and accurate detection, allowing for timely intervention and personalized treatment strategies.
Treatment
Treatment for congenital retinal dystrophies aims to slow disease progression, alleviate symptoms, and, in some cases, restore vision. While there is currently no cure for these conditions, various treatment options and emerging therapies provide hope for a better outcome.
Standard Treatment Options
- Vitamin and Antioxidant Supplements: Nutritional supplements like vitamin A and lutein have been shown to slow the progression of some retinal dystrophies. While the efficacy of these supplements varies, they can help improve overall retinal health.
- Low Vision Aids: Magnifying glasses, telescopic lenses, and electronic visual aids can help people with significant vision loss make the most of their remaining vision and maintain their independence.
- Occupational Therapy: Occupational therapists can help people adjust to vision loss and improve their ability to perform daily activities.
Surgical Interventions
- Cataract Surgery: When cataracts develop as a secondary complication of retinal dystrophies, cataract surgery can help improve vision by removing the clouded lens and replacing it with an artificial one.
- Vitrectomy: This surgical procedure removes the vitreous gel from the eye to treat complications like vitreous hemorrhage or macular hole, which can occur in the advanced stages of some retinal dystrophies.
Innovative and Emerging Therapies
- Gene Therapy: Gene therapy has shown promise for treating certain types of congenital retinal dystrophies. Luxturna (voretigene neparvovec-rzyl), an FDA-approved treatment for RPE65-associated Leber congenital amaurosis, delivers a functional copy of the RPE65 gene to retinal cells, which improves visual function. Similar therapies for other genetic mutations are currently being researched.
- Stem Cell Therapy: Stem cell therapy is the transplantation of healthy retinal cells derived from stem cells to replace damaged ones. Early-stage clinical trials are looking into the safety and efficacy of this approach for restoring retinal function.
- Retinal Implants: Retinal prostheses, such as the Argus II system, create artificial vision by converting visual information into electrical signals that stimulate the remaining retinal cells. Individuals with severe vision loss can benefit from these devices, as they can restore some functional vision.
- CRISPR/Cas9 Gene Editing: This cutting-edge technology enables the precise editing of specific genetic mutations. While still in the experimental stages, CRISPR/Cas9 has the potential to directly correct genetic defects that cause retinal dystrophies, potentially leading to a permanent cure.
- Pharmacological Treatments: New drugs seek to slow retinal degeneration or improve photoreceptor survival. Neuroprotective agents, anti-inflammatory drugs, and small molecules that target specific pathways involved in retinal cell death are being investigated.
Trusted Resources
Books
- “Inherited Retinal Disease: Diagnosis and Management” by Stephen H. Tsang
- “Retinal Dystrophies: Functional Genomics to Gene Therapy” edited by Bernard Weber, Reinhard Kellner, and Hendrik Scholl