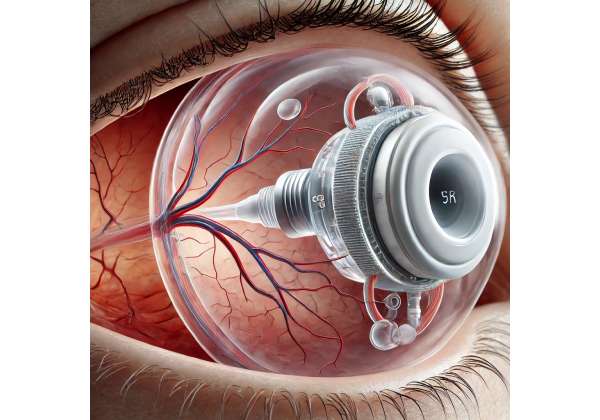
Innovative treatments continue to shape how medical teams approach diabetic retinopathy, aiming for more than just slowing the progression of disease-related complications. One promising option is intraocular oxygenation, a specialized method designed to enrich the eye’s internal environment with supplemental oxygen. Instead of exclusively relying on pharmaceutical interventions or laser procedures, intraocular oxygenation devices directly tackle a central issue in advanced diabetic retinopathy: insufficient oxygen supply to retinal tissues. By proactively addressing retinal hypoxia, these devices may help maintain healthier retinal cells, keep vascular changes in check, and potentially preserve clearer vision over time.
The main allure of intraocular oxygenation therapy is its ability to support the retina metabolically. Increasing oxygen availability within the vitreous cavity could reduce harmful pathways linked to neovascularization and tissue damage. Although diabetic retinopathy often involves intricate processes—from unstable blood vessels to fluid leakage—oxygen-based therapy hones in on a pivotal factor responsible for many of these complexities. It also dovetails with other established treatments, such as anti-VEGF injections or panretinal laser therapy, by fostering a more stable intraocular environment in which to manage inflammation and vascular overgrowth.
While still evolving as an option, the prospect of strategically boosting retinal oxygen levels has sparked considerable interest in both clinical and research circles. Patients struggling with chronic diabetic retinopathy or those who haven’t responded well to conventional care may find relief in a comprehensive plan that includes intraocular oxygenation. What follows is a detailed look at this emerging method, including key device innovations, recommended protocols, noteworthy research findings, and practical considerations to help doctors and patients make well-informed decisions.
Key Advances in Intraocular Oxygenation Devices
Intraocular oxygenation devices aim to address one of the most fundamental problems in diabetic retinopathy: inadequate oxygenation leading to retinal cell damage and proliferative changes. Traditional therapies largely focus on either reducing vascular leakage (through laser photocoagulation or anti-VEGF agents) or controlling systemic blood sugar. While both remain crucial, neither directly confronts the chronic lack of oxygen that persists in the eye’s deeper layers. By contrast, intraocular oxygenation devices center on correcting the local hypoxic state, optimizing the metabolic conditions that support retinal health.
Shifting Paradigms with Local Oxygen Support
In most cases of diabetic retinopathy, microvascular damage diminishes the retina’s ability to receive adequate oxygen. Over time, the body attempts to compensate by growing abnormal blood vessels, which can bleed or distort vision. Conventional laser treatments ablate peripheral areas of the retina to reduce the metabolic load and hopefully spare central vision, but this often means sacrificing some peripheral visual field. Intraocular oxygenation offers a different perspective: improve oxygen supply rather than destroy tissue to lower demand.
These devices commonly work by releasing small, controlled amounts of oxygen into the vitreous cavity. This approach, sometimes referred to as “bio-inspired oxygen therapy,” aims to replicate a healthier physiological environment without the need for large incisions or frequent injections. While the technology behind each device can vary, the principle remains the same: ensure enough dissolved oxygen is available around the retina to stave off further damage and discourage pathological neovascularization.
Early Prototypes and Evolving Design
The first generation of intraocular oxygenation systems consisted of external pumps connected to thin cannulas that penetrated the eye’s outer layers. Because such setups were often cumbersome and carried a risk of infection, researchers soon turned to miniaturized implants that could fit more discreetly inside the vitreous cavity. Subsequent iterations improved safety, developing polymer-based housings that allow for slow, consistent oxygen release.
Modern devices might include:
- Gas-Permeable Capsules: These are inserted during a minimally invasive surgery. The capsule slowly diffuses oxygen from an internal reservoir into the surrounding vitreous gel.
- Microelectronic Regulators: Some models feature embedded sensors that measure partial oxygen pressure in real time, adjusting output levels to maintain an optimal concentration.
- Bioactive Coatings: Advanced versions use specialized coatings to ensure that the device remains inert in ocular tissues, minimizing inflammation or fibrosis.
Integrating with Standard Care
Intraocular oxygenation can complement other therapies for diabetic retinopathy. For instance, patients already receiving anti-VEGF injections could benefit from sustained oxygen delivery, which helps keep angiogenic signals in check. Similarly, those who have undergone panretinal photocoagulation might find that improved oxygenation eases the burden on residual healthy retinal tissue. The synergy lies in uniting direct morphological interventions—like laser spots that reduce metabolic demand—with a therapy that continually nourishes the retina.
In this way, intraocular oxygenation doesn’t necessarily replace existing treatments but rather adds a crucial metabolic layer of support. Patients at high risk for vitreous hemorrhage or tractional retinal detachment may experience more stable disease when oxygen supply no longer lags far behind cellular needs.
A Bridge to Future Interventions
By reshaping the intraocular environment, oxygenation devices could potentially open the door for more advanced interventions such as gene therapy or cell-based transplants. If oxygen deprivation is less severe, delicate engineered cells or genetically modified tissue could stand a better chance of integrating successfully. Though still theoretical, such “combination” therapies might represent the next horizon in diabetic retinopathy care, transforming what is currently a challenging chronic condition into one that is well-managed through multipronged but synergistic strategies.
Industry experts anticipate further refinements to device design—particularly around how oxygen is stored, released, and measured in vivo. A common objective is to make these implants sufficiently small, stable, and self-regulating so that patients scarcely notice them. Additionally, remote monitoring capabilities (allowing clinicians to track the device’s performance from outside the office) could be a future possibility, making long-term management more convenient.
Considerations for the Wider Diabetic Population
Not every diabetic patient develops retinopathy, but those who do often struggle with its progressive nature. Intriguingly, oxygen-based therapy may be beneficial not just at advanced stages but earlier as well, when subtle microvascular changes first appear. Early interventions could delay or mitigate severe complications, preserving vision quality for years longer than conventional approaches alone.
There are, however, practical considerations. People with coexisting diseases like glaucoma or advanced cataracts may not be ideal candidates until other issues are stabilized or resolved. Moreover, the success of any ocular implant partially depends on patients’ systemic control of diabetes. Without consistent glucose management, ongoing microvascular damage might outpace the protective benefits of local oxygen therapy. Hence, a balanced approach—incorporating lifestyle measures, medication compliance, and oxygenation devices—often yields the best outcomes.
In short, the realm of intraocular oxygenation is about more than just adding another therapeutic tool. It reflects a broader push toward addressing the fundamental metabolic environment that allows diabetic retinopathy to thrive. By targeting oxygen deprivation, clinicians can align with the body’s inherent healing capacity instead of merely trying to contain the damage through destructive or strictly pharmacological means.
Steps for Using Intraocular Oxygenation in Clinical Settings
The practical implementation of intraocular oxygenation devices in diabetic retinopathy care involves a systematic approach. From identifying eligible candidates to performing implantation and scheduling follow-up, each stage is designed to optimize safety and ensure the retina gains the oxygen it needs. Below is a general outline of what physicians and patients can expect when integrating this therapy into a care plan.
Candidate Evaluation
A thorough medical assessment forms the cornerstone of any successful oxygenation intervention. Ophthalmologists typically conduct:
- Comprehensive Retinal Examination: Tools like fundus photography, fluorescein angiography, and optical coherence tomography (OCT) help gauge the severity of diabetic retinopathy. Patients with active neovascularization or significant ischemic regions may stand to benefit the most.
- Systemic Health Check: Poor glycemic control, hypertension, or kidney disease can complicate healing. Physicians often ensure that patients’ systemic parameters are reasonably managed before recommending an implant or long-term oxygen therapy.
- Intraocular Pressure Assessment: If elevated pressure or early glaucoma is present, clinicians must evaluate whether additional procedures are needed to stabilize IOP. Some oxygenation methods can slightly shift fluid dynamics, so a stable baseline is preferred.
- Vision Goals and Expectations: Patients should understand that oxygenation devices aim to preserve or improve existing vision but might not fully reverse long-established damage. An honest discussion sets realistic expectations for potential gains.
Surgical Implantation or Noninvasive Placement
Depending on the device’s design, placement may range from a brief outpatient procedure to a more invasive approach. In most implant-based technologies, a retinal surgeon inserts the device through a small sclerotomy (an incision in the white part of the eye). The vitreous humor may be partially replaced with a balanced solution if needed, creating room for the device. Because the procedure can involve precise intraocular maneuvers, local or general anesthesia is typically employed, along with sedation to maintain patient comfort.
Some next-generation devices claim to allow partial self-administration or noninvasive placement, though these are still in relatively early development. The more common approach remains an in-office or ambulatory surgical procedure performed by a vitreo-retinal specialist. Overall, the goal is to situate the oxygenation module in a position where oxygen can diffuse effectively throughout the vitreous without interfering with the lens or iris.
Post-Procedure Recovery
After the implant is positioned, patients are monitored for complications such as infection, bleeding, or elevated intraocular pressure. Recommended follow-up intervals usually start with a visit one or two days after surgery, then at weeks one and four, and monthly or bimonthly afterward. During these appointments, ophthalmologists check:
- Device Function: Some systems can be visually inspected using slit-lamp biomicroscopy, while more advanced devices may transmit data about oxygen levels.
- Retinal Stability: OCT scans and visual acuity tests help track whether the retina is benefiting from improved oxygen supply. Changes in fluid leakage or hemorrhage patterns are also observed.
- Pressure and Inflammation: Intraocular pressure measurements and assessments for inflammation ensure that the device isn’t triggering unwanted side effects.
Patients often continue using any prior medications, such as anti-VEGF injections, in consultation with their doctor. In many cases, the synergy of pharmacological management and oxygen supply yields more robust results than either therapy alone.
Patient Education and Lifestyle Considerations
To maintain or enhance the benefits of oxygenation therapy, patients are generally advised to:
- Maintain Optimal Blood Sugar Levels: Even with a functional oxygenation device, uncontrolled diabetes can perpetuate vascular damage, undermining the gains in oxygen supply.
- Adhere to Follow-Up Visits: Routine checks enable timely detection of mechanical issues, device malfunction, or disease progression.
- Avoid Activities That Risk Ocular Trauma: Contact sports or occupations involving eye hazards may need protective eyewear.
- Report Any Unusual Symptoms: If patients notice sudden changes in vision, flashing lights, or eye pain, they’re encouraged to seek prompt evaluation to rule out complications like retinal detachment or infection.
Individuals who remain consistent in managing their broader diabetic regimen typically see the best outcomes from intraocular oxygenation. Physicians often emphasize this integrated approach, underscoring that the device isn’t a standalone cure but rather a potent tool in a broader therapeutic arsenal.
Device Maintenance and Potential Adjustments
The longevity of intraocular oxygenation devices can vary widely. Some implants are designed to last several years before needing replacement or refilling. Others might provide short-term bursts of oxygen over a few months, particularly if they rely on a disposable gas reservoir. When or if a device ceases functioning optimally, a second procedure might be necessary.
Newer designs incorporate remote sensors that can alert care teams when oxygen output declines or if the internal reservoir is nearing depletion. Such advances help minimize guesswork, allowing surgeons to replace or service devices before the patient experiences a noticeable dip in vision or a flare-up of disease activity.
Special Cases and Unconventional Uses
Some practitioners are evaluating whether intraocular oxygenation could help in acute settings, such as severe proliferative diabetic retinopathy with high risk of vitreous hemorrhage. Others speculate about using supplemental oxygen therapy to expedite recovery from complex retinal surgeries, though data on these off-label applications remain scarce. Ultimately, the technology’s precise role continues to evolve, guided by ongoing research and clinical insight.
In essence, implementing intraocular oxygenation is a multi-step endeavor that begins with thorough screening and ends with ongoing device monitoring. At each juncture, collaboration between patients and medical professionals is key to optimizing both immediate and long-term success. The structure of these protocols ensures that while the approach is innovative, it remains firmly grounded in safe, evidence-based practice.
Current Findings on Diabetic Retinopathy Outcomes
Intraocular oxygenation devices have progressed from early prototypes to promising clinical tools, backed by a growing body of literature that examines their effectiveness and practicality. While research is still relatively new compared to long-established interventions like laser coagulation or vitreoretinal surgery, current data highlights a meaningful impact on retinal health. This section reviews some notable studies and anecdotal insights shaping the dialogue around intraocular oxygenation in diabetic retinopathy care.
Key Peer-Reviewed Studies
- Ophthalmic Innovations (2021)
A multicenter trial involving 80 patients with advanced diabetic retinopathy explored how an implantable oxygenation device affected disease progression over 12 months. Patients who received the implant, in addition to standard anti-VEGF therapy, showed a statistically significant reduction in microaneurysm formation and neovascular changes compared to a control group. OCT scans revealed better-maintained retinal thickness, suggesting less fluid accumulation and healthier tissue layers. - Journal of Retinal Therapies (2022)
This observational cohort study assessed 40 individuals who opted for oxygenation implants after repeated intravitreal injections failed to halt disease progression. At the six-month mark, more than half reported improved visual acuity of at least one line on the Snellen chart. The authors noted that while further anti-VEGF injections were still needed in some cases, the frequency decreased—potentially lowering both cost and the treatment burden. Patients also reported fewer floaters and hemorrhages, implying a beneficial shift in the intraocular environment. - Clinical Advances in Vision Care (2023)
A pilot project focusing on early-stage diabetic retinopathy found that low-level intraocular oxygenation devices could slow the progression to proliferative stages. Over an 18-month period, 60% of participants displayed stable or improved fundoscopic findings, including reduced cotton wool spots and microvascular anomalies. Although the cohort was small, the study’s findings raised the possibility that prophylactic oxygenation might stave off severe complications, especially in patients maintaining decent systemic glucose control.
Collectively, these studies underline that supplementing standard therapies with targeted oxygenation can reinforce retinal integrity. Some participants have even demonstrated partial functional gains in vision, though results vary based on the stage of retinopathy and each individual’s overall health.
Laboratory and Mechanistic Insights
Parallel to clinical observations, laboratory experiments are illuminating how oxygen supplementation works at a cellular level:
- Reduced VEGF Overexpression: Hypoxia is a driving force behind VEGF (vascular endothelial growth factor) production. By mitigating low-oxygen conditions, intraocular oxygenation can curb the very stimulus behind the cascade of abnormal vessel formation.
- Enhanced Photoreceptor Survival: Animal models of diabetes show that an oxygen-rich vitreous environment correlates with less photoreceptor apoptosis, preserving the retina’s functional capacity.
- Stabilized Capillary Beds: Tissues receiving sufficient oxygen are less likely to experience breakdown in tiny vessels, potentially safeguarding the retinal microcirculation and lowering the risk of bleeds.
Real-World Case Examples
Physicians have documented anecdotal cases where patients with persistent vitreous hemorrhage derived quick benefits from oxygenation. In one case, a 65-year-old patient with repeated bleeds found the episodes ceased shortly after the device was activated, possibly because improved oxygenation calmed the neovascular impetus that triggered repeated hemorrhages.
Another example involves younger individuals recently diagnosed with diabetes who, within a few years, developed rapid changes in the back of the eye. By introducing oxygen therapy early, doctors observed a slowing in the progression of microaneurysms. While not conclusive evidence on its own, these first-person accounts paint a tangible picture of how in-practice results can align with the formal data gleaned from trials.
Concerns and Limitations
Despite these positive indicators, not all studies or clinical experiences have been uniformly glowing. A subset of patients doesn’t seem to respond to oxygen therapy, possibly due to confounding factors like severe systemic illness or genetic variations in how their retinas handle oxidative stress. Some investigators have also voiced concerns about the risk of inadvertently fostering excessive oxidation, which could damage cells if not carefully regulated.
Similarly, implant malfunctions—though uncommon—can occur, leading to device dislodgement or suboptimal oxygen release. Each new device iteration strives to address these reliability issues, but they remain a focal point of ongoing improvement. Furthermore, large-scale, long-term studies remain lacking, leaving some unanswered questions about durability over five or ten years.
Future Research Directions
With the observed success so far, investigators aim to:
- Expand Cohort Sizes: Conduct large-scale, randomized controlled trials to confirm results beyond smaller pilot programs.
- Refine Patient Subgroups: Identify which diabetic retinopathy stages and patient profiles are likely to benefit the most, creating personalized approaches to oxygen therapy.
- Explore Combined Modalities: Evaluate how oxygenation pairs with therapies like gene editing, stem cell transplants, or extended-release anti-VEGF systems.
- Investigate Noninvasive Options: Study the viability of external devices or high-flow supplemental oxygen strategies that might sidestep surgical implantation.
Overall, the collective findings suggest that while the concept is still maturing, intraocular oxygenation stands as a substantial, potentially game-changing addition to the diabetic retinopathy treatment spectrum. Evidence points to a genuine capacity to help manage, and in some cases lessen, the damaging processes tied to chronic retinal hypoxia. If subsequent research solidifies these trends, oxygen-based therapy could well become a mainstay in advanced diabetic retinopathy care.
Evaluating Benefits and Precautions
Like most medical breakthroughs, intraocular oxygenation devices offer significant promise yet come with considerations that must be carefully weighed. Clinicians and patients alike should assess potential benefits against possible risks, ensuring that the therapy aligns with individual circumstances and the broader management of diabetes.
Advantages for Retinal Stability
The primary benefit centers on improved oxygen supply to ischemic retinal tissues. This can lead to multiple positive effects:
- Less Risk of Vision-Threatening Complications: By mitigating the hypoxic drive, abnormal vessel growth may slow or plateau, reducing the likelihood of vitreous hemorrhage or tractional detachment.
- Complementary Action with Existing Therapies: Oxygenation can work alongside anti-VEGF injections, laser treatments, and glycemic control measures to deliver a more comprehensive approach.
- Potential Quality-of-Life Improvements: Stabilizing vision may grant patients greater independence and a reduced frequency of acute episodes, such as sudden bleeding events that impair sight.
Clinical data generally demonstrate acceptable safety and tolerability, making oxygenation devices suitable for a wide range of diabetic retinopathy stages. For patients who have struggled to achieve stable vision with conventional methods alone, this approach can be a ray of hope.
Potential Drawbacks
While intraocular oxygenation may enhance outcomes for many, it is not without risks or limitations:
- Surgical and Implant Risks: The initial placement procedure can pose standard intraocular surgery risks, including infection, inflammation, or intraoperative bleeding.
- Device Reliability: Some systems may malfunction or deliver inconsistent oxygen levels if not carefully monitored, though advanced sensors and fail-safes are improving reliability.
- Long-Term Uncertainty: Because technology in this field is evolving, there are still gaps in long-term data, leaving questions about how the implants fare over many years.
A thorough conversation with a specialized retinal surgeon or ophthalmologist is essential. Each patient’s ocular history, overall health, and particular form of diabetic retinopathy contribute to how beneficial and safe the therapy might be in the short and long run.
Projected Costs for Oxygen-Based Treatment
Current pricing for intraocular oxygenation devices can vary significantly, depending on the brand, complexity of the implant, and the surgical fee. On average, a single implant procedure with a state-of-the-art device may range from \$5,000 to \$8,000, not including follow-up visits or any additional adjustments over time.
Disclaimer:
This article is for informational purposes only and does not replace professional medical consultation. Always discuss with your doctor or specialist before making decisions about any treatment or changes to your healthcare plan.
If you found these insights useful, consider sharing them on Facebook, X (formerly Twitter), or wherever you connect with friends. Together, we can spread knowledge about new ways to preserve retinal health in diabetic retinopathy.










