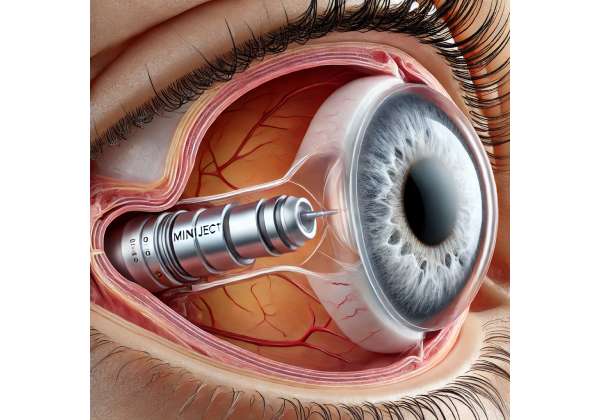
Glaucoma remains a leading cause of irreversible vision loss worldwide, prompting ongoing research into more refined and patient-friendly solutions. While established treatments—such as medicated eye drops, laser procedures, and surgical interventions—can help lower intraocular pressure (IOP), many individuals still seek better ways to preserve their sight with fewer side effects. This is where minimally invasive glaucoma surgery (MIGS) devices like Miniject come into play, offering an innovative approach that focuses on both efficacy and patient comfort.
Unlike certain procedures that involve extensive tissue manipulation, Miniject relies on micro-scale engineering to facilitate safer fluid drainage within the eye, targeting a reduction in the high pressures that often damage the optic nerve. By combining technological advances with a less invasive technique, Miniject has the potential to benefit those with mild-to-moderate open-angle glaucoma, and it continues to gain recognition among ophthalmologists looking to broaden their therapeutic options.
Exploring Miniject: A Closer Look at the Innovative Approach
Miniject is part of the growing MIGS category, a set of treatments designed to lower intraocular pressure without the complexity and risks associated with traditional surgeries like trabeculectomy or tube shunt implantation. At the heart of this device is a cutting-edge, porous material crafted to optimize fluid outflow from the anterior chamber of the eye to the suprachoroidal space. By establishing a controlled pathway for aqueous humor drainage, Miniject aims to reduce intraocular pressure effectively, lessening the load on the optic nerve and, in theory, slowing or halting the progression of glaucoma-related vision loss.
The Drive Behind Minimally Invasive Glaucoma Surgery
In conventional glaucoma surgeries, substantial disruption to ocular tissues often occurs, and complications such as infection, excessive scarring, or hypotony (overly low eye pressure) can arise. MIGS devices like Miniject were specifically developed to reduce these risks and complications. Instead of creating a major opening or drainage reservoir, a minuscule stent or implant is placed within the eye’s natural drainage pathways. This strategy not only speeds up recovery time but also lessens post-operative discomfort and makes follow-up care more manageable for patients and clinicians alike.
Moreover, from a patient-centered standpoint, MIGS technologies address the persistent problem of medication adherence. Eye drops—commonly used for daily glaucoma management—demand ongoing discipline, and any lapses in usage can lead to elevated IOP and potential disease progression. Minimally invasive procedures that deliver lasting pressure control help fill this gap by offering consistent outcomes without the constant worry about missing a dose.
What Makes Miniject Different?
Among the variety of MIGS devices, Miniject stands out for its flexible, soft, and biocompatible structure. Manufactured from a proprietary porous material, the device is designed to blend smoothly with the surrounding ocular tissues. According to laboratory data, this material fosters stable fluid flow patterns over time and resists the fibrotic reactions that sometimes hinder drainage in traditional implants.
A key element of Miniject is its suprachoroidal approach. Unlike trabecular meshwork-based MIGS devices that reroute fluid into the Schlemm’s canal or subconjunctival space, Miniject taps into the space between the sclera and the choroid. Studies have indicated that this region may be less prone to scarring and can accommodate an implant with minimal interference to the rest of the eye’s anatomy. Some ophthalmic specialists posit that suprachoroidal MIGS might prove especially valuable for patients whose disease is progressing despite typical medical or laser therapies.
Patient Suitability and Indications
Miniject typically targets those with primary open-angle glaucoma, one of the most common forms of the disease. In this subtype, the drainage angle remains open, but microstructural changes in the trabecular meshwork or elsewhere hinder aqueous outflow. The suprachoroidal device can help alleviate these obstructions by providing an alternate route for fluid egress.
Early and intermediate stages of glaucoma are generally the most promising for MIGS interventions, as these individuals often do not yet require the more aggressive surgical approaches. However, certain patients with advanced disease or complex ocular anatomies may also be candidates if they meet the criteria set forth by their ophthalmologist. In many instances, Miniject is combined with cataract surgery, allowing the device to be inserted through the same small incision used for lens removal. This synergy can streamline two procedures into a single operative session, optimizing the recovery timeline for patients with coexisting cataracts and glaucoma.
Longevity and Results
One of the biggest questions surrounding MIGS devices concerns their long-term durability. Research so far suggests that properly placed Miniject implants can remain effective over multiple years. Unlike stents made of metal or other rigid materials, the Miniject’s flexible design aims to reduce the likelihood of shifting or erosion, which could compromise outflow. Clinicians also monitor the eye’s natural healing processes and any fibrotic tissue formation that might eventually occlude the device. While continued follow-up studies are essential, preliminary data point to stable, enduring reductions in IOP for many individuals who receive Miniject.
Moreover, from a practical standpoint, Miniject’s insertion does not close the door on additional or alternative treatments. Patients who do not achieve adequate IOP control with the device can still consider other therapies, whether pharmaceutical or surgical. The minimally invasive nature of the procedure often leaves ocular structures sufficiently intact to accommodate subsequent interventions if necessary.
Potential Benefits for Different Patient Groups
- Older Adults: As people age, managing complex medication regimens can become more challenging. A single procedure that addresses both glaucoma and cataracts can reduce the burden on elderly patients while preserving vision.
- Active Professionals: Individuals who travel frequently or maintain a hectic schedule sometimes find it difficult to adhere to multiple eye drops daily. Miniject, as a one-time procedure with potential long-term benefits, may fit better into a demanding lifestyle.
- Early-Stage Patients Looking for Better Stability: Some patients opt for MIGS early in their disease to lessen reliance on daily medications and to gain more consistent control of their IOP.
Understanding the Risks
Even though Miniject is designed to lower complication rates, no surgery is entirely without risk. Potential complications include transient spikes in IOP, mild inflammation, or, in rare cases, suprachoroidal hemorrhage. However, published data generally show that these events are infrequent and often manageable through timely post-operative care. As with any MIGS device, patient selection is crucial, and thorough examinations by a qualified eye specialist can help predict the likelihood of favorable outcomes.
How the Procedure Works and Essential Treatment Steps
Once a patient is deemed a suitable candidate for Miniject, the operative strategy focuses on inserting the device into the suprachoroidal space through a small incision in the eye. The procedure usually occurs in an outpatient setting, allowing the patient to return home the same day.
Pre-Procedure Evaluations and Considerations
Before heading into surgery, patients often undergo a series of evaluations:
- Comprehensive Eye Exam: This step gauges the severity of glaucoma, measuring current IOP levels and assessing the optic nerve for signs of damage. Visual field tests and optical coherence tomography (OCT) scans offer a baseline for comparison after the procedure.
- Corneal Health Check: A healthy cornea is paramount for successful surgery. Conditions like corneal dystrophies or scarring could complicate the insertion or positioning of the device.
- Angle Assessment: Gonioscopy (examination of the drainage angle) is generally performed to confirm that the angle is open and to rule out angle-closure variants or peripheral anterior synechiae that might hinder the placement of the MIGS device.
- General Health Clearance: Certain medications or systemic conditions (such as clotting disorders) can affect surgical decisions. Blood tests and consultation with a primary care physician are standard for older or medically fragile patients.
In many instances, Miniject is implanted at the same time as cataract surgery, using a single corneal or scleral incision. This combination approach spares the patient from undergoing two separate procedures and optimizes operating room time.
Step-by-Step Surgical Process
- Anesthesia: Local or topical anesthesia is typically used to numb the eye, minimizing patient discomfort. Sedation may also be administered to help the individual relax.
- Incision Creation: A tiny incision—often just a few millimeters—is made near the corneal-scleral junction. If combined with cataract surgery, the same incision can serve both purposes.
- Device Preparation: The Miniject implant, preloaded on a specialized injector, is readied for insertion. Its soft, flexible design allows for smooth passage into the eye.
- Insertion Into the Suprachoroidal Space: Under microscopic visualization, the surgeon positions the implant between the sclera and choroid. This anatomical space is chosen for its ability to facilitate fluid outflow.
- Verification and Sealing: Once inserted, surgeons confirm proper positioning of the implant and ensure that the incision is watertight. Antibiotic and anti-inflammatory agents are typically applied to guard against infection and excessive inflammation.
The entire procedure can be relatively quick—often less than 30 minutes—though actual timing varies based on factors like the surgeon’s expertise and whether a cataract removal is performed concurrently.
Post-Operative Care and Follow-Up
After the procedure, patients usually remain in a recovery area for observation, where medical staff monitor their IOP and check for any immediate complications. Once they are stable, they can go home with a set of instructions that may include:
- Eye Drop Regimen: Although the device aims to reduce or eliminate the need for daily drops, initial anti-inflammatory or antibiotic drops are often prescribed to ward off infection and irritation.
- Activity Guidelines: Strenuous activities, heavy lifting, and direct eye contact (e.g., rubbing the eye) should be avoided for a certain period. A protective shield or patch might be used for the first day or so.
- Scheduled Examinations: Follow-up visits generally occur within the first week, the first month, and at subsequent intervals to track healing and measure IOP. If additional procedures or adjustments are required, the specialist can address them promptly.
Over time, many patients notice that their reliance on eye drops diminishes, although some may still need adjunct therapies to maintain adequate IOP control. Each individual’s response can vary, so personalized follow-up plans remain vital for ensuring the best long-term results.
Considerations for Combined Cataract Surgery
When Miniject is performed alongside cataract extraction, the synergy extends beyond incision-sharing. The same post-operative medication routine typically covers both procedures, streamlining recovery. Moreover, since vision improvement from cataract surgery is already a high priority for many older adults, pairing the procedures can deliver dual benefits: a clearer visual axis (courtesy of the new intraocular lens) and better IOP management through the suprachoroidal implant.
Potential Drawbacks and Limitations
- Intraoperative Challenges: Although less invasive than traditional surgeries, MIGS still demands careful technique. Surgeons must ensure that the device does not contact other critical structures in the eye.
- Variable Responses: Not every patient experiences the same degree of pressure reduction. Individual anatomical differences, scarring tendencies, and disease severity can influence outcomes.
- Need for Ongoing Monitoring: Even if the device offers substantial pressure relief, regular eye exams remain crucial. Glaucoma can progress subtly, and any sign of rising IOP or vision changes should be addressed promptly.
Overall, the procedure’s simplicity, combined with its potential for meaningful IOP control, underscores why Miniject has garnered attention as a promising therapy for open-angle glaucoma, especially when integrated with a broader treatment strategy that includes medication management and lifestyle considerations.
Key Studies and Emerging Findings from Clinical Trials
Miniject’s reception among ophthalmologists stems partly from compelling clinical evidence that suggests it can maintain lower intraocular pressures, reduce the need for additional medications, and do so with fewer complications than conventional surgical options. Several peer-reviewed studies, detailed in respected medical journals, have contributed to its growing credibility.
Multicenter Trial Evaluations
In an influential study published in the European Journal of Ophthalmology (2021), researchers conducted a multicenter trial across various European countries, evaluating Miniject implantation in patients diagnosed with mild-to-moderate open-angle glaucoma. Participants underwent a follow-up period spanning 12 months to assess changes in IOP, medication usage, and vision-related quality of life. Results indicated:
- Sustained Pressure Reduction: A majority of patients achieved IOP levels under 18 mmHg by the 6-month mark, with stable readings maintained through the final follow-up.
- Decreased Medication Burden: On average, individuals reduced their eye drop regimen from three daily medications to one, suggesting an appreciable improvement in convenience and adherence.
- Low Rate of Complications: Less than 5% of participants encountered serious issues like hypotony or device migration. Most side effects were minor and resolved spontaneously.
The study’s authors highlighted the device’s biocompatible material, noting minimal tissue reactions that could hamper drainage efficiency over time. By the end of the trial, many participants reported greater confidence in their ability to manage glaucoma, largely due to decreased dependence on daily medications.
U.S.-Based Pilot Studies
Although much of the initial data emerged from European clinical settings, more recent investigations have taken place in the United States. A pilot study featured in the Journal of Glaucoma (2022) recruited 40 patients from five different centers. Researchers aimed to gauge both surgical feasibility and six-month outcome measures. They found:
- Consistent IOP Control: Post-operative IOP averaged around 15 mmHg, a significant drop from baseline values that often hovered above 22 mmHg.
- Safety Profile: Adverse events were predominantly mild, including transient inflammation or short-lived pressure spikes. No patient required device removal.
- Suitability for Combined Procedures: About half of the participants also underwent cataract surgery, allowing investigators to evaluate the synergy between lens replacement and MIGS insertion. The results suggested that dual procedures did not compromise IOP outcomes and instead offered the dual benefit of refractive correction and glaucoma management.
A highlight of this pilot study was the emphasis on refining the surgical technique, underscoring how critical precise implant placement is in the suprachoroidal space. Surgeons reported a manageable learning curve, indicating that once proficient, the procedure could fit smoothly into standard surgical routines.
Long-Term Performance Insights
While short-term and intermediate results are promising, any new MIGS device must demonstrate long-term stability to gain widespread acceptance. Ongoing trials, including post-market surveillance studies, are crucial for confirming whether Miniject can sustain pressure control over multiple years. Early indications from a five-year follow-up extension—mentioned in a report presented at the American Academy of Ophthalmology’s 2023 conference—show that most patients continued to enjoy stable IOP management without additional surgeries or escalated medication usage.
In this extension study, a portion of participants experienced mild increases in IOP after the two-year mark, potentially linked to the eye’s natural healing process and scar formation. However, when mild laser touch-ups or topical drops were introduced, these individuals often regained pressure stability, suggesting that supplemental therapies can be effectively layered onto Miniject.
Comparative Data with Other MIGS Devices
Miniject is not the only MIGS option available. Other devices, like the iStent or XEN Gel Stent, also aim for minimized invasiveness and improved outflow. In a Comparative Ophthalmology Review (2022), authors examined different MIGS techniques to see how Miniject stacks up:
- Technique Variation: Each device targets a unique anatomical pathway. Trabecular bypass implants focus on Schlemm’s canal, while the XEN Gel Stent creates a drainage channel into the subconjunctival space. Miniject leverages the suprachoroidal route, which some surgeons believe may offer more consistent drainage.
- Head-to-Head Outcomes: While comprehensive comparative trials are ongoing, anecdotal evidence and small-scale analyses suggest Miniject’s suprachoroidal approach results in IOP reductions on par with—or in some cases surpassing—other MIGS devices.
- Safety Profiles: Across the MIGS spectrum, complication rates remain relatively low. Specific adverse events can differ based on the type of device. For Miniject, suprachoroidal hemorrhage is a theoretical concern, but documented cases are rare.
Given that MIGS devices vary in their design and targeted outflow pathways, personalized recommendations often hinge on a patient’s unique eye anatomy, disease stage, and likelihood of scarring. The ongoing accumulation of real-world data will further clarify which device best suits specific clinical scenarios.
Case Reports and Observational Data
In addition to formal studies, case reports in journals such as Ophthalmology Times have spotlighted individual successes. For instance, one report described a 67-year-old patient whose bilateral glaucoma had proven difficult to manage via eye drops alone. After Miniject implants in both eyes, they maintained stable pressures around 16–17 mmHg for over a year with significantly reduced medication load.
Observational data also abound in large clinics where practitioners have begun to incorporate Miniject into standard MIGS offerings. These anecdotal updates commonly emphasize how the device’s flexible design and suprachoroidal approach appear to generate favorable outcomes in a broad patient demographic, including those with prior ocular surgeries.
What Lies Ahead
As data from longer-term follow-ups accumulate, ophthalmic circles anticipate more robust guidelines on patient selection, pre-operative assessments, and post-operative care. Some researchers are also exploring how genetic factors or biomarkers might predict who responds best to suprachoroidal drainage. Additionally, ongoing refinements to the device’s insertion technique could simplify the procedure further, making it an even more appealing choice for busy surgical centers.
The consistent theme that emerges from current research is that Miniject has the potential to address a significant clinical need: achieving meaningful IOP reductions in a safer, less invasive manner than older surgical options. This sense of optimism is tempered by the acknowledgment that, like all emerging therapies, Miniject will continue to evolve with expanded clinical experience and evidence-based refinements.
Insights on Success Rates and Patient Safety
Miniject’s effectiveness varies from patient to patient, influenced by the severity of their glaucoma, their unique anatomy, and how well they follow post-operative care guidelines. Overall, published studies highlight a significant proportion of recipients achieving IOP levels under 18 mmHg, often with a reduced reliance on daily eye drops. Many retain these improvements for at least one to two years, and ongoing trials hint at the possibility of multi-year stability.
In terms of safety, complications like transient rises in intraocular pressure or mild inflammatory responses may occur in the initial weeks after implantation. More serious adverse events, such as suprachoroidal hemorrhage or device migration, are relatively rare based on the data currently available. Careful patient screening and skilled surgical technique stand out as pivotal factors in minimizing these risks. For those who do experience a less-than-ideal outcome, adjunct therapies or additional interventions can frequently restore pressure control.
Current Pricing and Insurance Considerations
Miniject’s cost largely depends on a variety of factors, including geographical location, the surgeon’s expertise, and whether the procedure is combined with cataract surgery. In some regions, the device and procedure fees can range between a few thousand and several thousand dollars, with potential additional expenses for anesthesia and facility use. Insurance coverage is gradually increasing, especially as more data confirm Miniject’s safety and effectiveness. Patients are encouraged to consult their ophthalmologist and insurance provider to identify possible reimbursements, co-pays, or financing arrangements that can offset out-of-pocket costs.
Medical Disclaimer:
This article is for informational purposes only and is not intended as a substitute for professional medical advice. Always consult a qualified healthcare provider for personalized guidance regarding glaucoma management and any associated treatments.
If you found this helpful, feel free to share it on Facebook or X (formerly Twitter) so others can learn about this minimally invasive glaucoma option.










