Eye Floaters After LASIK or Cataract Surgery: What’s Normal and What’s Not
Noticing new eye floaters after LASIK or cataract surgery can be unsettling, especially when your vision is otherwise clearer than it has been in...
Eye Makeup and Infections: How Often to Replace Mascara and Eyeliner
Eye makeup is meant to enhance your look, not compromise eye comfort. Yet mascara, eyeliner, and tools used near the lash line can become...
Eye Pain: Causes, Warning Signs, and When to Seek Urgent Care
Eye pain is a symptom, not a diagnosis—and it can range from a scratchy, surface-level sting to a deep ache that signals an emergency....
Eye Pain With COVID-19: Causes and Warning Signs
Eye pain during COVID-19 can be surprisingly intense—ranging from a scratchy burn to a deep ache behind the eye that makes light feel harsh....
Eye Pain With Influenza A: Causes, Red Flags, and What to Do
Eye pain during Influenza A can catch you off guard. One day it is a typical flu—fever, chills, body aches—and the next your eyes...
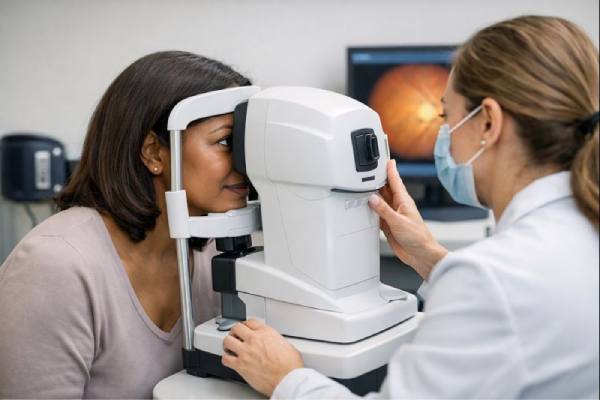
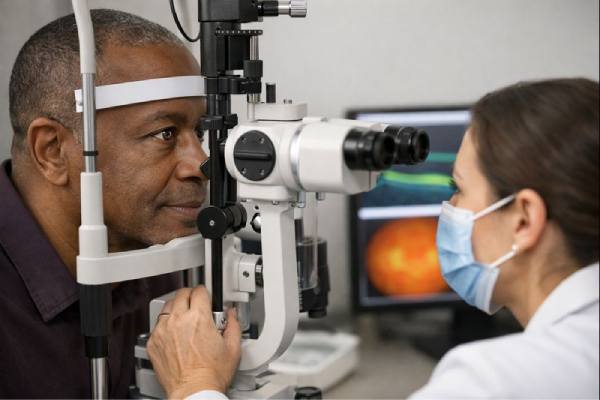
Eye Pressure Testing: What It Measures and What the Results Mean
Eye pressure testing is a quick measurement that helps clinicians estimate the pressure inside your eye, called intraocular pressure (IOP). It matters because pressure...
Eye Strain from Reading: Lighting, Font Size, and Better Setup
Eye strain from reading is usually less about “weak eyes” and more about a mismatch between your visual task and your environment: small text,...
Eye Strain Headaches: Screen Setup, Glasses, and Relief Strategies
Eye strain headaches are the kind that creep in during long reading or screen sessions—tight across the forehead, heavy behind the eyes, or paired...
Eye Twitching (Myokymia): Stress, Caffeine, and When It’s Not Normal
Eye twitching can feel oddly personal: a small flutter in the eyelid that shows up during meetings, while driving, or exactly when you are...
Eyelash Extensions and Eye Health: Irritation, Allergies, and Safety Tips
Eyelash extensions can be a confidence boost: they frame the eyes, reduce daily makeup time, and create a polished look that holds up through...
Eyelid Hygiene Routine: How to Clean Lids for Blepharitis and Dry Eye
Eyelid hygiene is one of the most practical, low-tech ways to improve blepharitis and many forms of dry eye—because the problem often starts at...
Flashes of Light in Vision: Causes, Risks, and When to Get Checked
Seeing flashes of light can be startling, especially when they appear without an obvious trigger. Many flashes are harmless and short-lived, but some are...
Gel vs Drops for Dry Eyes: Which Works Better and When to Use Each
Dry eye treatment can feel like guesswork until you understand one simple idea: not every dry eye needs the same kind of moisture. Standard...
Gene Therapy for Inherited Retinal Disease: Who Qualifies and What the Process Looks Like
Gene therapy is changing what “treatable” can mean for some inherited retinal diseases. Instead of managing symptoms alone, it aims to restore or improve...
Genetic Testing for Eye Disease: When It’s Worth It and How Results Guide Treatment
Genetic testing has changed what “an eye diagnosis” can mean. Instead of naming only what the retina or optic nerve looks like today, a...
Giant Papillary Conjunctivitis: Contact Lens–Related Itching, Mucus, and Fixes
Giant papillary conjunctivitis (GPC) is one of the most frustrating reasons contact lens wearers lose comfort: the eyes itch, strings of mucus appear, and...
Glaucoma Eye Drops: Types, Side Effects, and Common Mistakes
Glaucoma eye drops are designed to lower eye pressure (intraocular pressure, or IOP) to protect the optic nerve over years—not just days. For many...
Glaucoma Symptoms: Early Signs, Risk Factors, and When to Get Tested
Glaucoma is often called the “silent” cause of vision loss for a reason: the most common forms can damage the optic nerve for years...
Gritty Eyes: Causes, Dry Eye Links, and How to Fix It
That “sand in the eyes” feeling can be surprisingly persistent—worse in the morning, sharper at the end of a screen-heavy day, or triggered by...
Halos Around Lights: Causes, Treatments, and When to See a Doctor
Seeing halos around lights—rings, glow, or radiating haze around headlights and streetlamps—can be unsettling, especially at night. The encouraging part is that halos are...
Herpes in the Eye: Symptoms, Recurrence, and Treatment Options
Eye herpes is a viral infection that can affect the eyelids, the clear front window of the eye (cornea), and sometimes deeper structures. It...
High Blood Pressure and Vision: How Hypertension Can Affect Your Eyes
High blood pressure is often called a “silent” condition because it can damage blood vessels for years before you feel anything unusual. The eyes...
High Cholesterol and Eye Health: Arcus, Retinal Changes, and Risk Signs
High cholesterol is usually thought of as a heart issue, but the eyes can quietly reflect what is happening in your bloodstream. Certain eye...
How Often Can You Use Artificial Tears? Safe Frequency and Best Types
Artificial tears are one of the simplest ways to relieve dry, irritated eyes—yet many people are unsure how often they can use them safely....