Nonalcoholic fatty liver disease—now widely termed metabolic dysfunction–associated steatotic liver disease (MASLD)—is common, quiet, and tightly linked to cardiometabolic health. Most people never feel liver symptoms until late. That is why screening for fibrosis risk, not just fat in the liver, matters for longevity planning. A practical, stepwise approach—starting with simple blood tests and moving to noninvasive imaging when indicated—can flag who needs closer follow-up and who can focus on lifestyle without specialist care. This guide explains what to check, how to read results, when to escalate, and how often to come back to testing. If you are building a broader health strategy, see our companion resource on longevity biomarkers and tools for how liver screening fits with blood pressure, glucose, lipids, sleep, and fitness.
Table of Contents
- Who Should Screen and Which First-Line Tests to Use
- Reading Liver Enzymes in Context
- FIB-4 Components and Cut-Points (Conceptual Overview)
- Imaging Basics: Ultrasound vs Elastography
- How Often to Recheck Labs or Imaging
- When to Refer for Specialist Evaluation
- Tracking Progress: Waist, Labs, and Energy
Who Should Screen and Which First-Line Tests to Use
Screening is not about finding every person with liver fat. It is about identifying those at risk for advanced fibrosis—the stage that predicts future cirrhosis, complications, and earlier mortality. A general population screen is not recommended. Instead, focus on people with higher pretest probability:
- Adults with type 2 diabetes or prediabetes.
- Overweight or obesity, especially central adiposity or increasing waist size.
- Multiple metabolic risk factors (elevated triglycerides, low HDL-C, hypertension, insulin resistance).
- Persistently abnormal liver enzymes (even if “mild”).
- Known steatosis on any prior imaging.
- Family history of cirrhosis, or personal history of obstructive sleep apnea, polycystic ovary syndrome, hypothyroidism, or chronic kidney disease.
- People with “lean MASLD”—normal BMI but visceral adiposity, dysglycemia, or dyslipidemia.
First-line testing should be simple, inexpensive, and usable in primary care:
- Baseline blood panel: ALT, AST, platelet count, fasting glucose or HbA1c, lipid profile. Consider TSH if clinical context suggests hypothyroidism. Document alcohol intake clearly; quantify weekly grams.
- Calculate FIB-4 immediately from routine labs (age, AST, ALT, platelets). FIB-4 is a validated fibrosis risk score with strong negative predictive value. It is designed to triage who needs second-line testing.
- Consider secondary blood markers when available through your lab, such as Enhanced Liver Fibrosis (ELF). Use them as a second step after FIB-4 in indeterminate ranges.
- Reserve imaging for those with elevated or indeterminate FIB-4, or when clinical suspicion is high. Vibration-controlled transient elastography (VCTE, often called FibroScan) is the most common noninvasive test to estimate stiffness (a proxy for fibrosis). Conventional ultrasound is widely available, but its role is limited (see Imaging section).
Why this sequence? A staged pathway reduces unnecessary imaging and referrals while catching the people at real risk. It also keeps costs down and avoids overloading specialty clinics with low-risk cases. Document medications that can contribute to steatosis or enzyme elevation (amiodarone, tamoxifen, methotrexate, corticosteroids, certain chemotherapeutics), and look for alternative or concurrent liver disease when the presentation is atypical.
Practical starting plan
- Order ALT, AST, platelets, fasting glucose or HbA1c, and lipid panel.
- Compute FIB-4.
- If low risk by FIB-4, manage metabolic health and schedule periodic reassessment.
- If elevated or indeterminate, arrange elastography or a second-line blood test (ELF).
- If discordant results or high clinical concern, consider hepatology referral.
Reading Liver Enzymes in Context
ALT and AST are often the first clues that prompt a liver check, but they are imperfect on their own. Many people with significant fibrosis—sometimes even cirrhosis—have enzymes within the laboratory “normal” range. Conversely, transient flares can occur from illness, exercise, alcohol, or medications and do not equal progressive disease. The key is context and trend.
What “normal” means
Laboratory reference ranges often run high. In metabolic liver disease, stricter thresholds better reflect true normal. As a practical rule of thumb, consider ALT above ~30 U/L as abnormal in most adults, with lower typical limits in women. Persistent elevation over six months is more informative than a single blip. When values fluctuate, focus on the baseline trend rather than one outlier.
AST vs ALT patterns
- In MASLD/NAFLD, ALT tends to be higher than AST early on; as fibrosis advances, AST may catch up or exceed ALT.
- An AST\:ALT ratio >1 is not diagnostic but should prompt attention to fibrosis risk and alcohol history.
- Marked AST elevation with disproportionate ALT rise can suggest alcohol, muscle injury, or other etiologies.
Platelets matter
Thrombocytopenia (lower platelets) can signal portal hypertension and advanced fibrosis, but platelets are influenced by many conditions (infection, medications, immune disorders). In isolation they are not definitive, yet they are essential to FIB-4’s performance.
Common, fixable confounders
- Alcohol: Even “moderate” intake may compound risk, especially with central obesity or diabetes. Quantify intake carefully and reassess enzymes after a period of abstinence.
- Exercise: Strenuous workouts can transiently raise AST and ALT; retest after 5–7 days of lighter activity.
- Medications and supplements: Review for agents linked to steatosis or hepatotoxicity.
- Acute illness: Post-viral enzyme bumps are common; wait for recovery before risk stratification.
What to do with a “mildly high” ALT
If ALT is persistently above ~30 U/L (or above your lab’s threshold when clearly abnormal), calculate FIB-4 and evaluate metabolic risk. Do not rely on ALT to exclude disease; normal ALT does not rule out advanced fibrosis. If FIB-4 is low and there are no red flags, focus on metabolic optimization and recheck on a defined schedule. If FIB-4 is indeterminate or high, proceed to elastography.
Tie-in with glucose and lipids
Insulin resistance drives both liver fat and cardiovascular risk. When enzymes are borderline, pairing liver assessment with glucose markers clarifies the overall risk picture and often guides the first therapeutic moves—nutrition, weight reduction, physical activity, and sleep optimization.
Takeaway: Enzymes are a prompt, not a verdict. Use them to trigger FIB-4 and broader cardiometabolic assessment, and avoid overreacting to single measurements without context.
FIB-4 Components and Cut-Points (Conceptual Overview)
What is FIB-4?
FIB-4 is a simple equation that estimates the probability of advanced fibrosis using age, AST, ALT, and platelet count:
FIB-4 = (Age × AST) / (Platelets × √ALT)
It was validated across multiple liver diseases and performs well in MASLD for ruling out advanced fibrosis. Its strengths are cost, availability, and negative predictive value in primary care. Its limitations: performance degrades at age extremes; acute illness, alcohol, or nonhepatic platelet issues can distort results.
How to interpret cut-points (adult primary care setting)
- Low risk: FIB-4 < 1.3 → Advanced fibrosis unlikely. Manage in primary care with lifestyle and cardiometabolic risk control.
- Indeterminate: FIB-4 1.3–2.67 → Needs second-line testing (usually elastography) or a specialty blood test (e.g., ELF).
- High risk (rule-in likely): FIB-4 ≥ 2.67 → Advanced fibrosis likely. Confirm with elastography and consider specialist referral.
Age adjustments
In adults >65 years, FIB-4 tends to rise with age independent of fibrosis. Many pathways use a higher “rule-out” threshold—commonly <2.0—to avoid false positives in older adults, while keeping ≥2.67 as a rule-in signal. Still, clinical judgment is essential: some recent analyses argue that 1.3 remains useful even in older groups, whereas others support the age-adjusted approach. The safest course is to apply FIB-4 alongside clinical suspicion and follow with elastography when results are borderline.
When FIB-4 can mislead
- Under 35 years: Accuracy is lower; lean, young adults with metabolic risk may need elastography despite a low FIB-4.
- Acute inflammation or heavy training week: AST/ALT spikes can drive FIB-4 up temporarily. Retest when well.
- Low platelets from nonhepatic causes: Immune thrombocytopenia or myelosuppression can inflate FIB-4.
Why FIB-4 first, not ultrasound first?
FIB-4 targets fibrosis risk—the outcome that matters most for prognosis—whereas ultrasound primarily detects fat and can miss riskier patients with normal-appearing livers. A two-step approach (FIB-4 → elastography if needed) reduces missed advanced fibrosis and streamlines referrals.
Connection to broader inflammation
Systemic inflammation can nudge AST/ALT and platelets. In indeterminate cases, markers like high-sensitivity CRP from inflammation testing may add context, but they do not replace fibrosis-directed tools.
Bottom line: Use FIB-4 to triage. Respect the thresholds, repeat when the clinical picture changes, and confirm with elastography when FIB-4 is indeterminate or high.
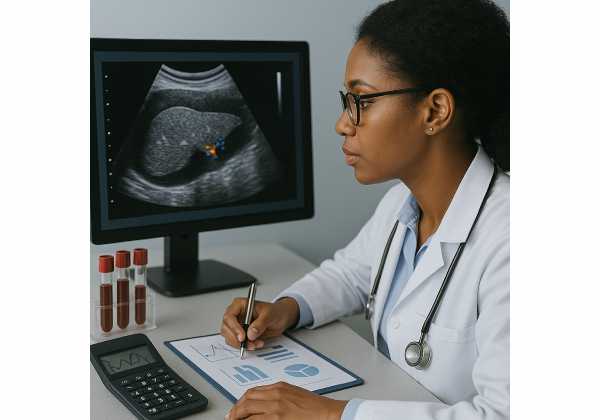
Imaging Basics: Ultrasound vs Elastography
Conventional abdominal ultrasound
Ultrasound is widely available and inexpensive. It detects steatosis when fat content is moderate to high, but sensitivity drops when fat is mild or in people with higher BMI. It also cannot grade fibrosis reliably. Artefacts from bowel gas, body habitus, and operator variability make subtle changes hard to track. In modern pathways, ultrasound is not recommended as a stand-alone tool to diagnose or quantify liver fat for risk stratification. That said, it still has a role: it can identify gallstones, masses, biliary dilation, or other structural issues that need attention, and in resource-limited settings it may be the only accessible modality.
Vibration-controlled transient elastography (VCTE, FibroScan)
Elastography estimates liver stiffness in kilopascals (kPa) as a proxy for fibrosis. It is painless, quick, and can be performed at the point of care. Typical interpretation for MASLD (numbers vary by device, probe, and context):
- Low probability of advanced fibrosis: often <8 kPa.
- Indeterminate range: roughly 8–12 kPa.
- High probability of advanced fibrosis: >12 kPa (higher values raise concern for cirrhosis).
Important caveats
- Probe selection: The XL probe improves accuracy in obesity.
- Biologic confounders: Active inflammation, cholestasis, and recent heavy alcohol use can temporarily increase stiffness. Fasting for 2–3 hours and avoiding vigorous exercise beforehand improves reliability.
- Repeatability: Use the same device and probe type when monitoring.
Controlled Attenuation Parameter (CAP)
Many VCTE devices report CAP (dB/m) to estimate steatosis. CAP is useful for tracking directional change in liver fat but does not define risk the way stiffness does. Treat CAP as supportive information, not a standalone decision maker.
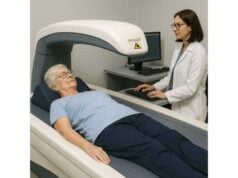
Magnetic resonance–based tools
- MRI-PDFF quantifies liver fat with high precision and is excellent for research and for evaluating treatment response, but cost and access limit routine use.
- Magnetic resonance elastography (MRE) provides highly accurate fibrosis assessment and can clarify discordant cases. It is usually reserved for specialty settings.
Putting it together
A pragmatic pathway in primary care is: calculate FIB-4 → if ≥1.3, perform elastography (or ELF if elastography access is limited) → refer or monitor based on combined results. Ultrasound may still be ordered alongside this pathway for general abdominal evaluation, but it should not replace fibrosis-directed testing.
Body composition affects imaging
Because higher BMI influences both ultrasound quality and elastography, pairing imaging with a structured view of body composition can help you interpret results and plan weight-loss targets.
How Often to Recheck Labs or Imaging
Rechecking too often adds noise; waiting too long misses change. The cadence depends on baseline risk, comorbidities, and whether you are actively intervening (nutrition, weight loss, medication changes).
If FIB-4 is low risk
- No diabetes and few metabolic risks: Reassess FIB-4 every 2–3 years.
- Prediabetes, multiple metabolic risks, or steatosis on prior imaging: Reassess every 1–2 years.
If FIB-4 is indeterminate (1.3–2.67)
- Perform elastography (or ELF) to clarify risk.
- If elastography suggests low stiffness and your clinical suspicion is low, repeat FIB-4 in 6–12 months while you optimize lifestyle and metabolic health.
- If elastography is borderline, tighten follow-up to 6–12 months and consider early specialist input.
If FIB-4 is high (≥2.67) or elastography is clearly elevated
- Confirm with elastography if not already done.
- If advanced fibrosis is likely or confirmed, transition to hepatology for staging, surveillance planning, and metabolic therapy; monitoring intervals become individualized (often every 6–12 months initially).
During active lifestyle or medication changes
- After meaningful weight loss, improved glycemia, or alcohol reduction, enzymes may improve within weeks and stiffness over months. Recheck ALT/AST and FIB-4 at 3–6 months to capture directionality, then space out to your long-term cadence.
Avoid “false urgency”
Do not recheck within 4–8 weeks of an acute illness, vaccine reaction, heavy training week, or alcohol binge—these can transiently raise enzymes and stiffness, muddying interpretation.
Integrate with cardiometabolic monitoring
Align liver follow-ups with existing check-ins for blood pressure and lipids. If you are already reviewing home logs for blood pressure tracking or renewing statins, add liver labs to that cadence.
Signals to accelerate the timeline
- Rising FIB-4 across two measurements.
- New thrombocytopenia, AST>ALT trend shift, or worsening insulin resistance.
- New symptoms: abdominal distention, leg edema, jaundice, right upper quadrant discomfort, pruritus, or easy bruising.
Bottom line: Pick a schedule that matches risk, avoid noisy retesting after short-term perturbations, and synchronize with broader cardiometabolic care.
When to Refer for Specialist Evaluation
Primary care can manage most low-risk MASLD, but timely referral prevents missed advanced disease and speeds treatment for those who need it.
Refer promptly when any of the following are present
- FIB-4 ≥2.67 or elastography >12 kPa (or clearly elevated per your lab/device cutoffs).
- Persistent enzyme elevation >6 months without a clear explanation.
- Discordant tests: Low FIB-4 but high elastography (or vice versa), or a mismatch between labs and the clinical picture.
- Possible alternative or concurrent disease: viral hepatitis, autoimmune hepatitis, hemochromatosis, Wilson disease, drug-induced liver injury.
- Signs of advanced disease: low platelets, splenomegaly, coagulopathy, or symptoms of portal hypertension.
- Pregnancy planning with high-risk findings or presence of significant alcohol use disorder.
What hepatology adds
- Confirmation of staging with advanced imaging or specialized blood tests (ELF, MRE).
- Management of cirrhosis complications and hepatocellular carcinoma surveillance when indicated.
- Consideration of pharmacotherapies as they become available.
- Guidance on complex medication choices when there are comorbidities such as CKD or autoimmune disease.
Do not wait on referral to start risk reduction
Begin lifestyle and cardiometabolic therapy immediately: nutritional counseling, physical activity plans, sleep hygiene, and alcohol reduction. Optimize atherogenic lipids with ApoB-focused therapy when indicated; cardiovascular events remain the leading cause of death in MASLD.
Documentation that speeds specialty care
Include weight history and waist measures, medication list (flagging potential hepatotoxic agents), alcohol intake quantified in grams/week, viral hepatitis serologies if available, and copies of elastography reports (with probe type and interquartile range).
Bottom line: Use firm thresholds, clinical judgment, and the “if in doubt, escalate” rule. A brief referral note with the right data accelerates answers.
Tracking Progress: Waist, Labs, and Energy
Your goal is not a “perfect” liver number. It is a healthier trajectory that aligns with longer healthspan: less visceral fat, better insulin sensitivity, lower atherogenic lipids, stable energy, and sustainable habits. Translate test results into a simple dashboard you can act on.
1) Waist and weight trend
Waist circumference and waist-to-height ratio track visceral fat better than the scale alone. Aim for a waist-to-height ratio <0.5 in most adults (context matters for height, sex, and ethnicity). Even 5–10% weight loss meaningfully improves steatosis; ≥10% can improve steatohepatitis and fibrosis in many people. Recheck waist every 4–8 weeks and log progress.
2) Enzymes and FIB-4
- ALT/AST should trend down with effective interventions, though plateaus happen.
- FIB-4 may improve more slowly; look at direction over time, not single points.
- If numbers stall, reassess adherence, sleep, medication side effects, and alcohol.
3) Elastography
If baseline stiffness was elevated, repeat after 6–12 months of consistent changes to confirm improvement. Use the same device and probe when possible.
4) Diet pattern
Sustained calorie deficit matters most for weight loss. Diets higher in fiber, nonstarchy vegetables, legumes, and unsaturated fats and lower in refined starches and added sugars often improve glycemia and triglycerides. Adequate protein (about 1.0–1.2 g/kg/day for many adults; adjust for kidney disease) helps preserve lean mass during weight loss.
5) Physical activity
Target 150–300 minutes/week of moderate aerobic activity plus 2–3 weekly resistance sessions. On weeks of heavy training, remember transient AST/ALT bumps are common; avoid over-interpreting a single high reading.
6) Sleep and alcohol
Prioritize 7–9 hours of consistent sleep. Alcohol is synergistic with metabolic stress; if enzymes are elevated or fibrosis risk is present, abstinence or near-abstinence is the safest path.
7) Cardiometabolic co-management
Coordinate with your clinician on glucose, blood pressure, and lipids. Statins are safe in MASLD and reduce cardiovascular risk. Omega-3 therapies (including icosapent ethyl) lower triglycerides in high-risk patients; they are not liver cures but support the broader risk picture.
A weekly check-in approach
- Mon: Weigh in and measure waist.
- Midweek: Log training sessions and energy levels.
- Fri: Plan next week’s meals and grocery list.
- Monthly: Review enzyme trends; adjust as needed.
Mindset
Expect gradual change. Aim for routines you can keep when life gets busy—simple meal templates, scheduled walks, home resistance options, and a short sleep wind-down ritual. Your labs will reflect these systems.
References
- AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease 2023 (Guideline)
- EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) 2024 (Guideline)
- EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update 2021 (Guideline)
- AGA Clinical Practice Update on the Role of Noninvasive Biomarkers in the Evaluation and Management of Nonalcoholic Fatty Liver Disease: Expert Review 2023 (Guideline)
- Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis 2021 (Systematic Review)
Disclaimer
This article is for educational purposes and is not a substitute for professional medical advice, diagnosis, or treatment. Always consult your clinician for personalized recommendations, especially before changing medications, supplements, or treatment plans. If you have symptoms of advanced liver disease—jaundice, abdominal swelling, confusion, or gastrointestinal bleeding—seek urgent medical care.
If you found this useful, please consider sharing it on Facebook, X (formerly Twitter), or any platform you prefer, and follow us for future updates. Your support helps us continue creating practical, high-quality health content.